experiment 6: introduction to methods for the isolation, quantification and analysis of DNA
1/161
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
162 Terms
why is isolation and purification important
fundamental preliminary requirement for detailed studies and understanding of the structure, properties and function of any biological molecule
if the molecule was not pure
a researcher could no know what proportion of a signal is coming from the molecule under investigation, and what potion is coming from other contaminating molecular species.
the typical sequence of steps for the purification of a molecule can be described as;
1. cell lysis
2. isolation of molecule of interest
3. detection and measurement of molecule of interest
cell lysis
-usually a single step intended to produce access to the internal cell contents
isolation of molecule of interest
set of processes where the molecule one wants to purify is physically separated from other molecules originally present in the cell.
detection and measurement of molecule of interest
-a way to evaluate how effective a purification process is.
-you figure out how much of the molecule you have
what is cell lysis
break open the cell to release cellular contents such as organelles, cytosol, dissolved salts and any molecules or molecular complexes that are present in the cytoplasm into an aqueous mixture
lysate
a preparation containing the products of lysis of cells
ways to lyse cells
-can bed one in a variety of physical or chemical techniques
-includes grinding in a mortar and petle
-repeated cycles of freeze/thaw using liquid nitrogen
-sonification by high frequency sound waves
-membrane solubilisation by detergents or high concentrations of salts such as guanidine hydrochloride.
-can combine these methods to ensure cell is lysed.
how to lyse bacterial cells
if attempting to lyse bacteria cells such as E. coli, enzymatic degradation of the cell wall by the enzyme lysozyme can be used.
how to lyse plant cells
more vigorous measures may be required as the cell wall must also be broken open.
structural fragility
-many biomolecules have structural fragility.
-care must be taken to ensure that the lysis step does not irrevocably degrade the molecule of interest which would otherwise make it impossible to obtain useful structural or functional information about it.
in what kind of solution should you lyse a cell into
purify molecule in solution that mimics the conditions under which the molecule is found in the cell
-this promotes stability
what pH should you lyse a cell in
-use a pH buffer that preserves the correct pH.
temperature to lyse a cell in
lysis can be performed at room temperature or at 4 degrees C.
-depends on susceptibility to degradation of molecule of interest
temperature of purification of nucleic acids
-can be performed at room temperature because the harsh conditions of cell lysis which damage cellular structures and denature nucleic-acid degrading enzymes will leave nucleic acids intact or in a state from which their native structure can be easily recovered.
temperature of purification of proteins
-ideally performed at 4 degrees C to minimize any deterioration of the protein.
-since the disruption of tissues and breaking open of cells frequently releases proteases into the lysate, (remember proteases are enzymes that cut proteins into smaller pieces), controlling the negative effects of this is important.
-the cooler temperatures reduce the activity of the proteases and frequently coctails of protease inhibitors are also added to the lysate to block or dramatically decrease their activity.
-once the proteases have been removed at an early stage of the purification process. this is no longer concern.
selective isolation of molecule of interest
when one prepares a cell lysate one essentially has all the thousands different types of molecules present in the cell mixed together in a relatively disordered way, and the objective of course is to find some process, or set of processes that can be used to separate the molecule that one wants from all the other molecules present.
techniques to separate molecules based on their difference in
1. solubility
2. charge
3. mass and shape
4. polarity
5. affinity for select ligands
chromatography
protein purification methods that exploit some of these properties
-it is the name given to a broad class of techniques that separate molecules in a complex mixture.
-the types of molecules that can be separated by chromatographic techniques is highly varied and depends upon the specific form of chromatography used.
what is the most commonly used methods for chromatographic separation of proteins
-size exclusion chromatography (SEC)
-ion exchange chromatography (IEX)
-high performance liquid chromatography (HPLC)
-capillary electrophoresis (CE)
-these are relatively non-specific separation method and do not always efficiently isolate different molecules with different physiochemical properties.
the 1 general purification method that is relatively molecule specific
affinity chromatography
affinity chromatography
-based upon reversible biological-type affinity interactions
-since biological interactions can be high specific, techniques that mimic or exploit this specificity can be used to quickly isolate a specific molecule.
centrifugation
relies upon difference in solubility and/or mass as the basis for separating different molecules.
historical nucleic acid extraction and purification procedures
utilized potentially hazardous organic solvents with complicated, delicate procedures that were difficult for less experienced researchers to master.
what do we use now for nucleic acid extraction and purification procedures
commercially prepared kits that have simplified and standardized the extraction and purification of nucleic acids and this makes it relatively easy for novice experimenters to obtain good yields of pure, high quality molecules.
how do the kits work
after the cells have been lysed, the kits work by selectively degrading and precipitating non-nucleic acid biological molecules which are removed by centrifugation or by washing them off a column.
columns for nucleic acid purification
-usually silica-based and nucleic acid molecules will bind tightly to these columns under certain ionic conditions - this allows for the removal of residual contaminants without significant loss of nucleic acid.
-the nucleic acids can then be eluted out of the column by altering the salt and pH conditions in the column.
-at this point they can be used immediately for further investigations or stored at -20 degrees C until required.
different types of nucleic acids
genomic, organelle and plasmid DNA.
-additionally, there is also numerous types of RNA present in cells (rRNA, mRNA, tRNA etc.)
-although all of these molecules will have different sequences of nucleotides or ribonucleotides, there is considerable structural similarity between different molecular species which presents considerable challenges if you are trying to isolate one particular type of molecule.
challenge of separating nucleotides
there are ways to separate broad classes of different types of nucleic acids from each other, but it can be very difficult to separate closely related molecules with similar nucleotide sequence (considerably increases the cost).
what do you do if you want a specific nucleotide sequence
-you use the techniques of molecular biology and cloning to manufacture the product you want.
electrophoresis
-allows for fairly specific and effective separation of nucleic acids
-method for separating charged molecules in an electric field.
-the technique can be used to physically separate different nucleic acid molecules depending upon their size.
most common electrophoresis for separating different nucleic acids
agarose gel electrophoresis
agarose gel electrophoresis
-can be used to partially purify nucleic acids because after you run the nucleic acid sample on a gel, you can physically cut the piece of gel that contains you desired nucleic acids from the gel and then purify it further from the gel itself using spin-column technology
agarose gel electrophoresis drawback
if there is a different nucleic acid of a size similar to the one you want, you will not be able to separate them from each other as they will both migrate to the same place on the gel.
detection and measurement of molecule of interest
-if one is attempting to purify a molecule, you need some sort of method to ascertain how much of the molecule that you want is present at each step of the purification process.
-helps the researcher evaluate the effectiveness of any particular step of the overall purification process.
-ex. a researcher could find that a particular step does not increase the purity of the molecule which means that step should be abandoned as it is just a waste of time and resources.
what are the easiest molecules to detect and quantify
enzymes
-this is the case if an established assay specific for the enzyme is already available.
-the reason for this is because in these situations only the specific enzyme generates a measurable signal (ie. the formation of product) and any other types of molecules that are still around will not (ideally) interfere with measurement.
specific activity
the usual approach to quantify purity of enzymes
what is specific activity
-the ratio of amount of enzyme activity that is present in a sample to the amount of protein (ie. your protein and any contaminating protein that is present).
units of specific activity
umol/min/mg of protein
-the 2 separate measurements need to be made in order to establish the specific activity of a sample.
umole/min component
determined from measuring the enzyme activity
mg of protein component
this comes from the standard protein assay
an increase in the specific activity during the purification process
indicates that the amount of enzyme relative to the amount of protein is increasing (the purity is also increasing).
-specific activity reaches a maximum for any particular enzyme when the only protein that is present is the enzyme, ie. you have a pure enzyme
2 ways to calculate specific activity

enzyme assay
-add a fixed volume of purification product to an excess of enzyme substrate and then the amount of product that is formed over time is measured with a spectrophotometer
-the amount of product that will be formed is proportional to the amount of enzyme that is present in the assay cuvette.
-since you know how much of your purification product you added, you can easily calculate how much enzyme is present in that sample.
protein challenge
-quantifying protein content generally is not difficult, but determining the content of a specific protein in a protein mixture can be a challenge.
reason
protein quantification methods are generic and detect some attribute (peptide bonds, aromatic residues etc.) common to most proteins
-when you measure the protein content of a mixture of proteins you do not know how much of the measured signal is from other contaminating proteins rather than the particular protein you are trying to purify
-it is possible to quantify a specific protein within a complex protein mixture, but to do so relies upon the use of expensive and potentially laborious techniques.
lab methods
-antibodies can be engineered to bind to only a specific protein after which the amount of bound antibody can be quantified which will allow the determination of the target protein.
-the protein sample can be digested with the protease trypsin, and the resulting protein fragments analyzed by mass spectrophotometric based techniques which can provide a detailed picture of the protein composition of the mixture.
-these methods are not suitable for practical routine protein purification.
the most common way to evaluate the purity and quantity of a protein preparation
-sodium dodecyl sulfate polyacrylamide gel electrophoresis.
-SDS PAGE
-this method separates different proteins based upon the mass of their polypeptide chains on a porous gel under the influence of an electric field.
bands in SDS PAGE
-if different sized proteins are present in the sample (ie. it is not pure) multiple bands, or a smear will appear on the gel when it is visualized
-a pure sample should only produce a single band (this assumes that a multimeric protein consists of polypeptide chains that are of equal size)
intensity of band
-this method can also be used to quantify the protein as the intensity of any particular band of protein on the gel will roughly correspond to the amount of protein.
difficulties in identifying and quantifying nucleic acids in a complex micture
-traditional methods rely on detecting structural elements common to most nucleic acid molecules.
-now it relies on fluorescent probes that uniquely bind to specific sequence, and this requires the synthesis of custom-designed and manufactured molecules which are generally too expensive for routine laboratory use.
how to quantify nucleic acids
-simply purify and measure its absorbance at 260 nm
Physics of centrifugation
in solution, particles have tendency to sink to bottom because of gravity. speed is affected by size and density.
-if the container is swung in a circle, the particles will sink faster due to the fact that the forces acting on the particles are greater than that of gravity.
centrifugation
-the most basic technique of spinning a mixture of particles at extremely high speeds.
-this subjects the particles to extremely large centrifugal forces which has the effect of separating some of the particles in the mixture from each other depending upon the intrinsic properties of the particles themselves.
centrifuge
machine that separates substances by whirling them
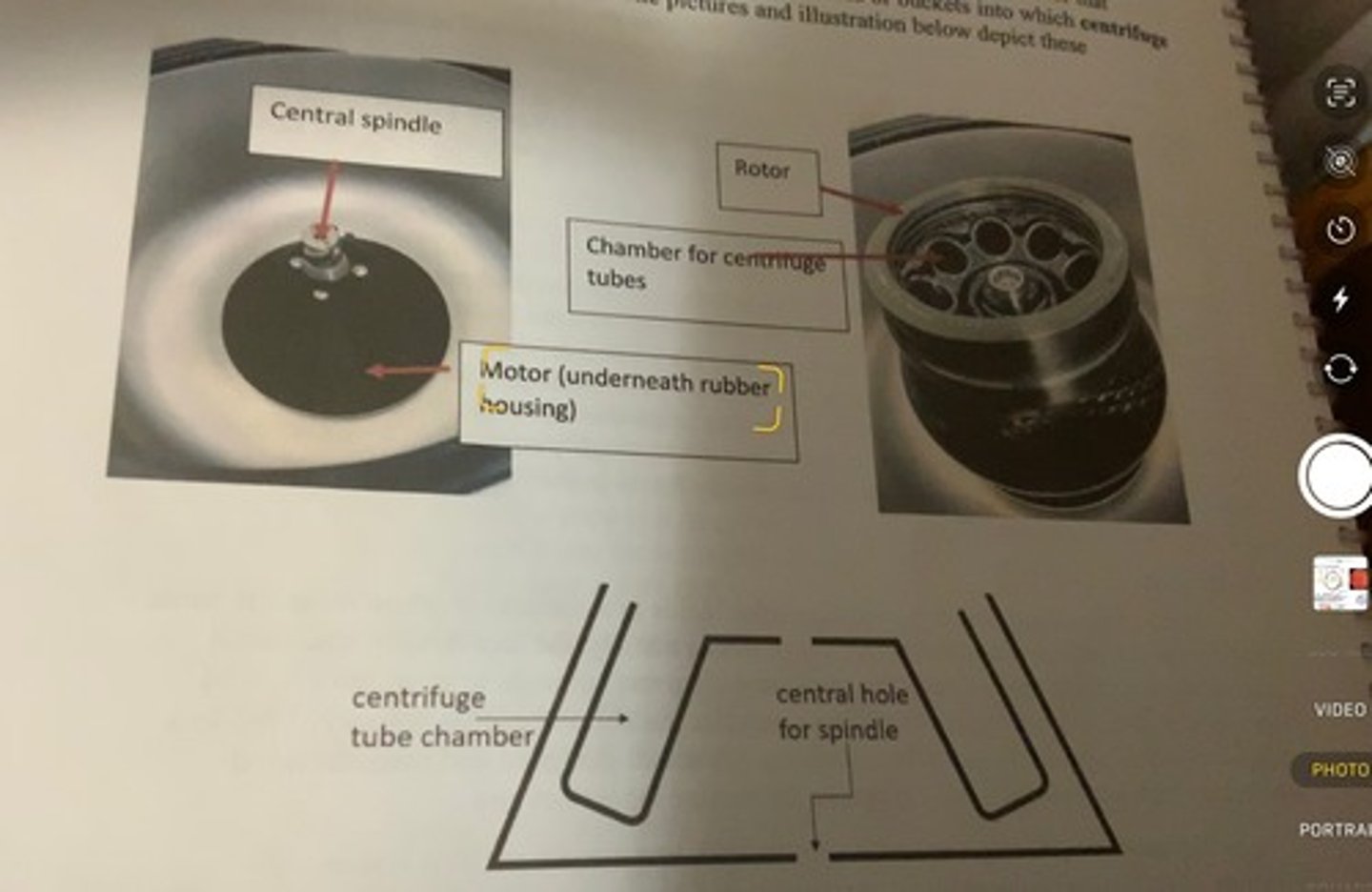
components of a centrifuge
-most centrifuges share common design features: namely a motor that rotates, a central spindle attached to the motor that spins when the motor is rotating, and a rotor that attaches to the central spindle.
-the rotor will contain chambers or buckets into which centrifuge tubes that contain samples are placed.
theory of centrifugation
the centrifugal force that is generated when the centrifuge is spinning samples can be quantified in multiple ways, and the most commonly used method is to define it as the relative centrifugal force (RCF)
-this approach allows for the easy quantification of this force and more importantly, a comparison of the forces generated by different centrifuges with different rotor sizes.
units of RCF
g
-with the notation "x g", representing multiples of the force of the earth's gravity.
RCF equation
-rpm = revolutions per minute at which the rotor is spinning about the central axis
-r = average distance the centrifuge tube is from the axis of rotation in cm.
-it is always important to report centrifugation steps in a protocol as RCF rather than rpm, as the force being generated will depend upon the size of the rotor, and not just the speed at which it is spinning.

centrifugal force (F)
-the actual force exerted on specific particles in a centrifuge tube when the rotor is spinning .
-m = effective mass of the partical
-w = angular velocity in rad/second
-r = distance of the particle from the centre of the axis of rotation
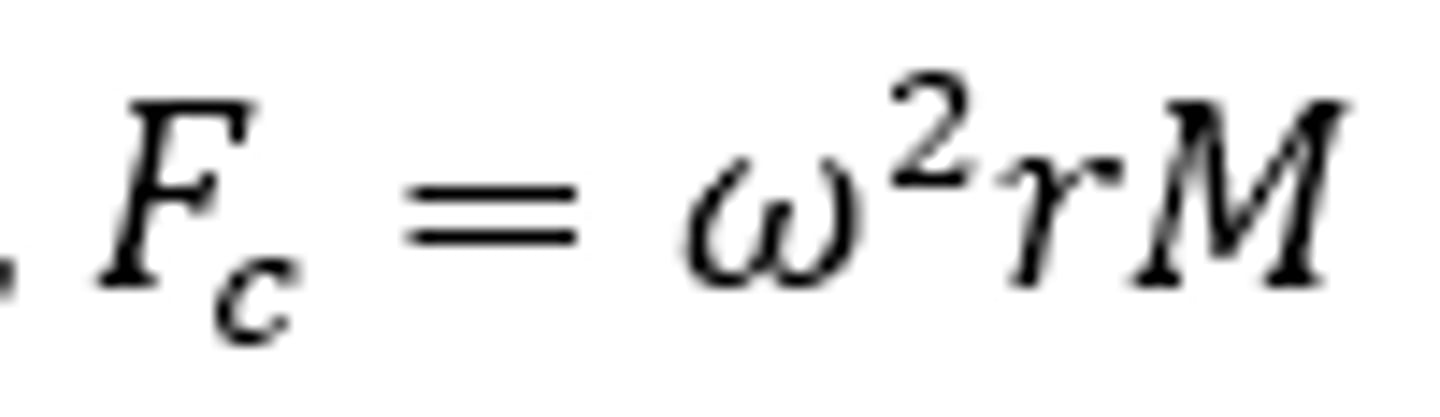
how is the effective mass similar to the actual mass
it is adjusted to account for the displacement of the particle in a solvent, and the density of that solvent.
why can a centrifuge be used to separate various particles in a mixture
-the force experienced by the particle is closely related to the mass of the particle.
-many particles will have different masses, and therefore will experience different forces and will move towards the bottom of the centrifuge tube at different speeds.
effective force is not the only factor that determines the speed at which a particle will move when subjected to a large centrifugal force.
-as soon as it begins moving, it will encounter a frictional force generated by its movement through the solvent molecules.
-this frictional force will work against the centrifugal force accelerating the particle.
what is the magnitude of the frictional force affected by
-by the size and shape of the particle and the speed at which it is moving
frictional force formula
f = frictional coefficient
v= velocity of the movement of the particle in the solvent
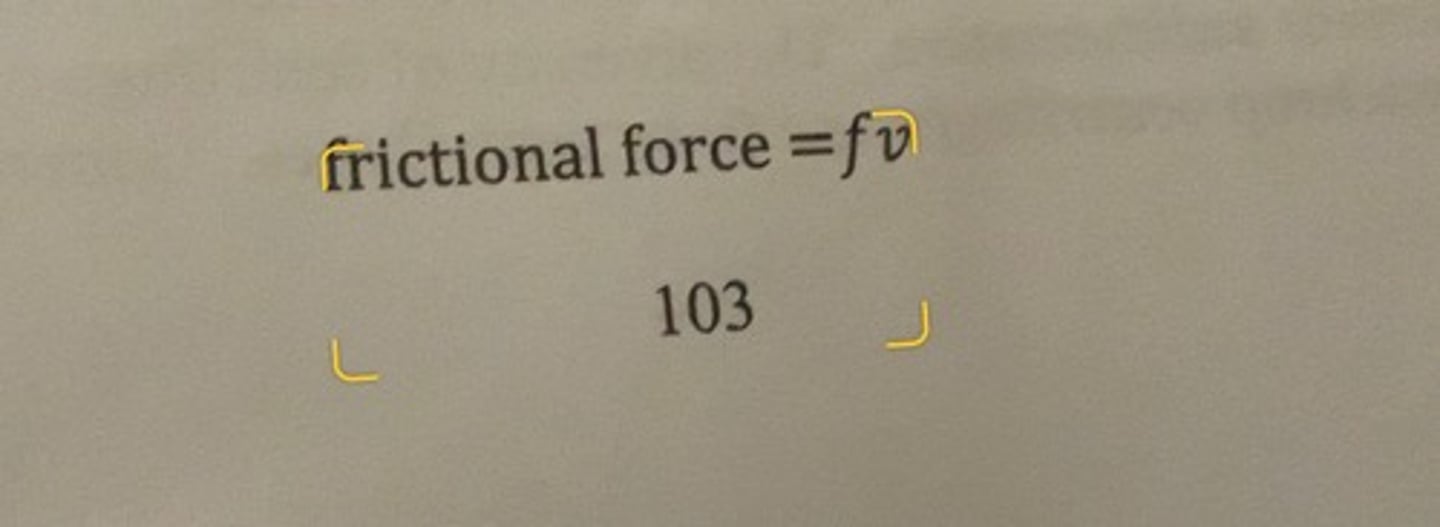
frictional coefficient
-complex parameter that takes into account the size and shape of the particle, and the viscosity of the solvent.
large particle
will experience a greater frictional force because it has to move through more solvent molecules.
sphere
moves more efficiently through a solvent compared to a flat sheet.
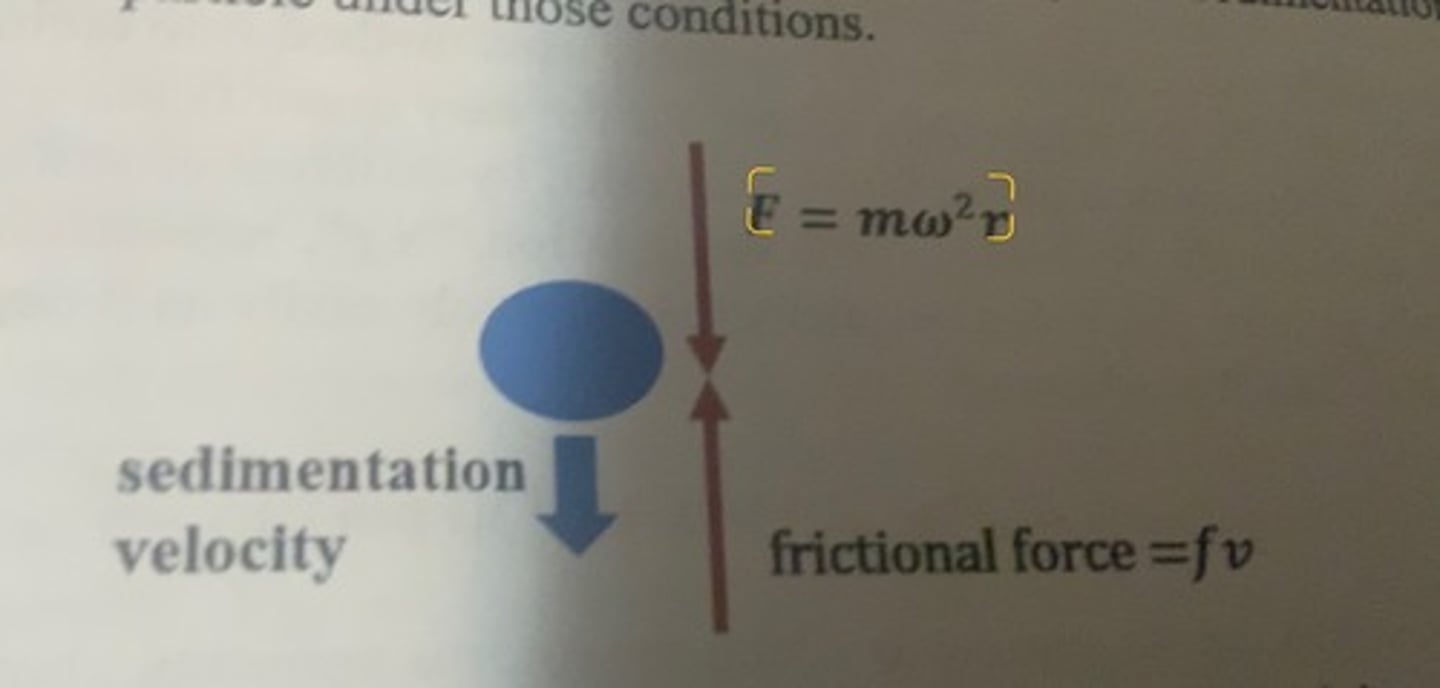
F = f
-after the rotor has been spinning at any particular rpm, the centrifugal force and then frictional force will equalize and a particle will migrate at a steady state sedimentation velocity specific to that particular particle under those conditions.
larger particles
-will generally have greater mass and will experience a greater centrifugal force
-larger particles will have a larger frictional coefficient and a greater friction force counteracting the centrifugal force.
does this mean particles of different sizes travel at similar speeds?
-no
-the mass of the particle is closely related to the volume of the particle.
-as the size of the particle increases, surface area increases more slowly than the volume which effectively means that the centrifugal force experienced by a more massive particle increases faster than the frictional force its larger size generates, and therefore larger particles will move faster than smaller particles.
sedimentation coefficient (s)
-has units of Svedberg
the larger the sedimentation coefficient
the faster it migrates in the centrifuge tube,
-any particle will have a sedimentation coefficient.
larger particles will generally have a ----------- sedimentation coeficient
larger
types of centrifuges
there are many types available.
-they vary with maximum volumes of sample they can accommodate, the max speed they can rotate and availability of refrigeration capability, the types of rotors used, and the availability of optical systems to monitor sedimentation.
the 2 classes of centrifuge types
1. benchtop centrifuges
2. floor centrifuges
benchtop centrifuges
-moderate RCF capability
-smaller volumes
floor centrifuges
-used for large volume preparations and/or moderate to high RCF requirements.
-from left to right: traditional bench top centrifuge, microfuge and mini centrifuge

traditional benchtop centrifuges
-typically accommodate 8-12 centrifuge tubes that have volume capacities ranging from 15-50 mL each.
-these centrifuges are capable of maximum RCF values of approximately 25,000 x g.
-some models from some manufacturers are capable of achieving maximum RCF values of 50,000 x g, but these are relatively uncommon.
models with refrigeration capability
they are available and relatively common as many labs will use these benchtop centrifuges for protein preparative purposes which are ideally conducted at 4 degrees C.
micro centrifuges (microfuge)
-will typically accommodate 24 microfuge tubes (1.5-2 mL each)
-primarily used for small-scale preparative work.
-capable of maximum RCF values of 25,000 x g.
-models with refrigeration capability are also available, but many labs have room temperature only models as the most common uses for microfuges do not require refrigeration.
-most nucleic acid purification kits are designed for these centrifuges.
mini centrifuge
-accommodate 4-8 microfuge tubes and are designed for quick low-speed spins such as driving liquids to the bottom of the tube or sedimenting large precipitates.
-have maximum RCF of several hundred g.
-almost never refrigerated.
what are floor model centrifuges used for
for large scale and/or analytical procedures.
-very expensive
-many floor centrifuges are compatible with a variety of rotors and because different rotors accommodate different sized centrifuge tubes, this provides considerable flexibility for the researcher.
-the range of sizes of centrifuge tubes that different types of rotors can hold varies from 50 mL up to 1.5 L.
-all floor model centrifuges have refrigeration capability.

what are floor models designed for
large scale preparative work
what is the maximum RCF of floor centrifuge
100,000 x g.
ultracentrifuge
-a second type of floor model centrifuge
-capable of extraordinarily high RCF values
-all have capability to generate a vacuum in the chamber in which the rotor rotates.
maximum RCF in ultracentrifuge
elite ultracentrifuges can attain maximum RCF values in excess of 1 million x g.
why is the vacuum a necessity
due to the extremely high rpm rates that must be attained to achieve such RCF values
-without a vacuum, the high rate of rotation of the rotor would generate excessive heat that would be destructive to the centrifuge.
in ultracentrifuge, how long does it take to evacuate the air in the rotor chamber
-1-2 hours to evacuate the air in the rotor chamber prior to starting a run.
analytical ultracentrifuges
-subclass of ultracentrifuge
-they include optical accessories and use special rotors that allow for the continuous monitoring of sedimentation of an analyte during a centrifugation run.
rotors
rotors come in different sizes
-can accommodate centrifuge tubes ranaging from less than 1 mL up to 1.5L.
the 3 major classes of centrifuge rotors
fixed angle rotors
horizontal or swinging bucket rotors
vertical rotors (uncommon)
what is the most common type of rotor
fixed angle rotors.
-come in the widest variety of sizes.
why is it called fixed angle rotor
the centrifuge tube sits at an unchanging angle (typically 25-40 degrees) in the rotor and the pellet will form in one corner and up the edge of the centrifuge tube during centrifugation.
what kind of centrifuges use fixed angle rotors
mini-centrifuges and ultracentrifuges
swinging bucket rotors
-the centrifuge tubes sit in a bucket that is in turn attached to the rotor at only 2 points which allows the buckets to swing out in the rotating plane of the rotor when the rotor is spinning.

what are swining bucket rotors used for
-for more specialized applications such as density gradient centrifugation
-can be used for traditional centrifugation purposes.
-due to the fact that the slots into which the centrifuge tubes are inserted in the bucket are small, the volumes that these types of rotors can handle is less than fixed angle rotors. if you have a large number of smaller centrifuge tubes, a swinging bucket rotor can allow you to run more tubes in a single centrifuge run which is useful
-available for both benchtop and floor model centrifuges.
the applications of centrifugation can be subdivided into 2 general categories
preparative and analytical
preparative application
exploit the fact that different molecules, structures and cells have different sedimentation coefficients which can be used for purification purposes.