AP Chem Unit 1
1/70
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
71 Terms
proton
symbol: p⁺ location: nucleus charge: +1 mass: 1amu
neutron
symbol: n⁰ location: nucleus charge: 0 mass: 1amu
electron
symbol: e⁻ location: energy levels (shells) around nucleus charge: -1 mass: 1/1840amu
atomic mass
mass of an atom, measured in atomic mass units (AMU) official definition: 1/12 of a carbon-12 atom
atomic mass unit
measurement used for atomic mass
moles
used to measure atoms, because they are very small and can't be counted
top left number in a nucleotide symbol:
the mass number (protons + neutrons)
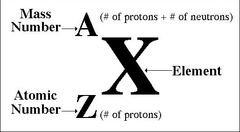
bottom left number in a nucleotide symbol:
the atomic number (protons)
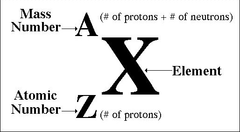
mass number
protons + neutrons
top right number in a nucleotide symbol:
charge of element protons-electrons
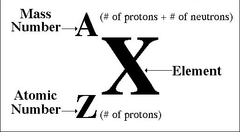
1 mole=
6.022 x 10²³ particles/formula units
particles
atoms, molecules, or ions
formula units
used for ionic bonds
molar mass
average mass of one mole of a substance in grams (g/mol) for one mole, there are this many grams
average atomic mass
the weighted average of the naturally occurring isotopes for a given element measured in AMU
average atomic mass formula
AAM= (m₁ * %₁) + (m₂ * %₂) + ... (mₙ * %ₙ) remember to change all percentages to decimals!
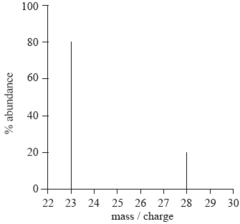
mass spectrometry
scientific method to determine the mass of atoms-relative abundance/AAM of samples
gives the mass-to-charge ratio
separates isotopes according to mass
the relative size of the peaks indicates the relative number of particles
the number of peaks=number of isotopes
pure substances
elements and compounds (molecules) are chemically bound together and need a chemical reaction to separate
mixtures
A combination of two or more substances that are not chemically combined, can physically separate
law of definite proportions
elements and compounds can only combine in simple, whole number ratios
if the ratio is different, then the sample is no longer pure and is a mixture instead
2 experiments to determine purity
reactions + stoichiometry
elemental analysis + empirical formula
elemental analysis
one of the experiments to help determine purity
the experimental measurement of the percent composition of elements in a compound
empirical formula
chemical formula showing the smallest whole-number ratio of atoms in a compound
how to do empirical formula
assume your sample is 100 grams, and change the percentages to grams
convert the grams to moles through dimensional analysis
divide all the molar amounts by the last number of moles
should be close to a whole number--if not, multiply
the numbers become the subscripts in the empirical formula
molecular formula
the true, unreduced formula for the atoms in a compound
how to do molecular formula
calculate the mass of the empirical formula
divide the compound's mass by the mass of the empirical formula (big over small)
multiply the subscripts by the number calculated in step 2
alkali metals (4)
group 1 on periodic table
most reactive metal family
react violently with water
often bind with halogens
alkaline earth metals (2)
group 2
hydroxides of these provide basic solutions in water
chalcogen family (2)
group 16
found in metal ores
halogen family (4)
group 17
known as salt formers
used in modern lighting
often bind with alkali metals
noble gas family (3)
group 18
known for their lack of reactivity
once thought to never react
atomic radius trend (4)
how large the atom is
increases down and to the left
down: because of increasing energy level--the more energy shells, the further electrons are from the nucleus and the larger the atom
across: because the effective nuclear charge increases to the left with less protons, causing it to have less coulombic attraction which doesn't pull electrons in as close
coulombic attraction
The force of attraction between positive and negative charges
ionization energy trend (5)
the energy it takes to remove an electron from an atom in the gas phase
only first ionization energy
increases up and to the right (opposite of atomic radius)
up: because the smaller the atom is with less energy shells, the smaller the atomic radius is and the closer together the electrons are, making it harder to remove from the nucleus
right: because the more protons there are, the more coulombic attraction there is which makes it harder to remove an electron
it always takes ___ energy to move more electrons
more
when is there a big change between ionization energies?
when it switches to removing core electrons
notable ionization energy trend exceptions (2)
between groups 2 and 13
between groups 15 and 16
groups 2 and 13 ionization energy trend exception (2)
happens because there is a difference in s and p orbitals
the s orbital has more ionization energy
groups 15 and 16 ionization energy trend exception (2)
happens because there is a difference in paired and unpaired electrons
it take less energy to remove a paired electron than unpaired
cations (3)
positively charged ions (cats are positive)
lost electrons
smaller than their atom because: the remaining electrons experience more attraction from the nucleus, making the radius smaller, and if you lose an electron, you have less electron-electron repulsion
anions (3)
negatively charged ions (onions are negative)
gained electrons
larger than their atom because there is more electron-electron repulsion
isoelectric
things with the same number of electrons
focus on the number of protons!
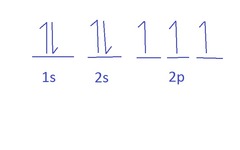
orbital notation/Aufbau diagrams
visual method that shows the arrangement of electrons in an atom
how to do orbital notation/Aufbau diagrams
write down electron configuration if needed
draw blanks for each orbital, with 2 electrons going in each blank (1 for s, 3 for p, 5 for d)
draw arrows, with up arrows in each orbital going first and down arrows second
Pauli exclusion principle
No 2 electrons in an atom can have the same set of four quantum numbers
no atomic orbital can contain more than 2 electrons. - To occupy the same orbital, they must be of opposite spin
Hund's Rule
the most stable arrangement of electrons is that with the maximum number of unpaired electrons because it minimizes electron-electron repulsions.
all single electrons have parallel spins to reduce electron-electron repulsions.
spread out than fill up
s level orbitals
1
p level orbitals
3
d level orbitals
5
f level orbitals
7
s level # of electrons in each orbital
2
p level # of electrons in each orbital
6
d level # of electrons in each orbital
10
f level # of electrons in each orbital
14
center of the atom is _____ charged
positively
electrons in energy shells are ____ charged
negatively
protons and electrons are ____ to each other
attracted
Coulomb's law
states the relationship between charge of particles and the distance between them
directly related to the charges of the particles (q)
inversely related to the distance between the particles (r)
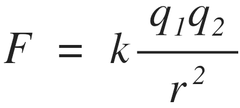
inner electrons have a _____ binding energy than outer electrons because:
greater, because of coulombic attraction
binding energy
amount of energy it takes an electron to leave
photoelectron spectroscopy (5)
provides data about ionization energy and its applications
gives electron energy data
amount of energy tells you about the electron's location--higher energy=closer to nucleus
relative size of peaks indicates the relative number of electrons
consistent with electron configuration
electronegativity trend (3)
ability of an atom in a molecule to attract electrons to itself
increases up and to the right, no noble gases (same as ionization energy)
because: shielding effect; also smaller atoms more easily feel charge of nucleus
shielding effect
if a molecule is small, there are less energy shells in the way, so more electrons can be attracted to it--explains electronegativity trend
electron affinity (4)
amount of energy given off when an electron is added to an atom
opposite of ionization energy
electron affinity trend
increases to the right because those atoms want another electron a lot (more negative), give off more energy
elements in the same ____ react the same way with other compounds
group
elements in the same group react the same way with other compounds because (3)
they have the same number of valence electrons
they have the same ending to their electron configurations
they have the same oxidation number
valence electrons
electrons in the outermost energy levels of an atom
core electrons
electrons not in the outermost energy level of an atom
oxidation number
most common ion forming for a given element
based on the number of valence electrons
goal is to get a full valence shell (8)
nonmetals _____ ____ electron shells, metals _____ _____ electron shells
fill up, remove outermost