Chapter 8 - Bonding - AP Chemistry
1/99
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
100 Terms
a chemical bond is
Forces that hold groups of atoms together and make them function as a unit.
A bond will form if the energy of the aggregate is lower than that of the separated atoms. (basically if making a bond is exothermic, it will form)
No simple, and yet complete, way to define this
The 4 Key Ideas in Bonding
Ionic Bonding – electrons are transferred
Covalent Bonding – electrons are shared (either equally or unequally)
Metallic Bonding - “sea” of mobile electrons
Network Covalent - special/unusual covalents made of long chains of elements, usually C or Si
Covalent Network Solids are a
special group of covalently bonded molecules or atoms which consist of atoms that are covalently bonded together into 2-D(sheets) or 3-D networks
Formed from nonmetals
Elemental (same element throughout C)
2 nonmetals (ex: SiO2 - quartz or SiC)
Unusually high melting/boiling points and are very hard (ex. graphiite and diamond)
An ionic compund is
any compound that conducts an electric current when melted will be classified as ionic.
why do ionic compounds conduct electric currents when melted? → Because the ions become separated (rigid solid to a free moving liquid) and are able to move along a charge.
A polar covalent bond is
Unequal sharing of electrons between atoms in a molecule.
Results in a charge separation in the bond (partial positive and partial negative charge).
The Effect of an Electric Field on Hydrogen Fluoride Molecules:
δ+ or δ- indicates a positive or negative fractional charge
all the fluorines face the positive side while all the hydrogens face the negative side since they’re attracted to the opposite charge
Electronegativity is
The ability of an atom in a molecule to attract shared electrons to itself.
For a molecule HX, the relative electronegativities of the H and X atoms are determined by comparing the measured H–X bond energy with the “expected” H–X bond energy.
Electronegativity the Trend:
On the periodic table, electronegativity generally increases across a period and decreases down a group.
The range of electronegativity values is from 4.0 for fluorine (the most electronegative) to 0.7 for cesium and francium (the least electronegative).
Noble gases are assumed to have electronegativity values of 0
Trend is due to the atomic radius trend. A smaller radius has a shorter distance between the nucleus and the outermost electrons so potential for attraction is higher
If lithium and fluorine react, which has more attraction for an electron? Why?
Fluorine, because it has the highest electronegativity value on the PT
In a bond between fluorine and iodine, which has more attraction for an electron? Why?
Fluorine, because it has the highest electronegativity value on the PT
Electronegativity difference thresholds:
Less than 0.5 = nonpolar covalent
Between 0.5 and 1.9 = polar covalent
Higher than 1.9 = ionic/extremely polar covalent
Arrange the following bonds from most to least polar: N - F, O - F, C - F
C - F, N - F, O - F
Which of the following bonds would be the least polar yet still be considered polar covalent?
Mg–O
C–O
O–O
Si–O
N–O
The correct answer is N-O. To be considered polar covalent, unequal sharing of electrons must still occur. Choose the bond with the least difference in electronegativity yet there is still some unequal sharing of electrons.
Which of the following bonds would be the most polar without being considered ionic?
Mg–O
C–O
O–O
Si–O
N–O
Si - O
Greatest END that doesn’t contain a metal
A Dipole Moment is..
a property of a molecule whose charge distribution can be represented by a center of positive charge and a center of negative charge
Use an arrow to represent a dipole moment
Point to the negative charge center with the tail of the arrow indicating the positive center of charge
Identify the Bond Polarity (BP) and the Molecule Polarity (MP) for each of the following: Draw Lewis Structures to help with symmetry analysis
CH4
SeCl2
SiS2
O3
CH4: BP is nonpolar due to small END and MP is nonpolar due to molecule being symmetrical and no lone pair(s) of electrons on central atom
SeCl2: BP: Polar due to END being somewhat large. MP: Polar due to lone pairs of electrons on the central atom making it asymmetrical
SiS2: BP: Polar due to END being somewhat large. MP: Nonpolar due to symmetrical molecule and no lone pair(s) of electrons on central atom. The bond dipoles perfectly cancel
O3: BP: Nonpolar because END = 0. MP: Polar due to lone pair of electrons on central atom making it asymmetrical. (Reminder that O3 requires resonance).
Some tips to evaluate polarity are:
C-H bonds are classified as being nonpolar bonds (END is very low)
If there’s lone pair(s) of electrons on the central atom, the MP (molecule polarity) is likely polar due to asymmetry.
Exception to this rule: expanded linear and square planar geometries as they are symmetrical even with their lone pairs of electrons on central atom. Their dipoles perfectly cancel (but only if the bonded atoms are identical)
In Stable Compounds atoms usually have a…
noble gas electron configuration
Electron Configurations in Stable Compounds
When two nonmetals react to form a covalent bond, they share electrons in a way that completes the valence electron configurations of both atoms.
When a nonmetal and a representative-group metal react to form a binary ionic compound, the ions form so that the valence electron configuration of the nonmetal achieves the electron configuration of the next noble gas atom. The valence orbitals of the metal are emptied and achieve the electron configuration of the previous noble gas atom
An isoelectronic series is…
a series of ions/atoms containing the same number of electrons.
What properties can the periodic table predict?
Atomic size, ion radius, ionization energy, electronegativity
Electron configurations
Formula prediction for ionic compounds
Covalent bond polarity ranking
What are the factors that influence the stability and the structures of solid binary ionic compounds?
How strongly the ions attract each other in the solid state is indicated by the lattice energy.
Lattice Energy is…
the change in energy that takes place when separated gaseous ions are packed together to form an ionic solid
LE = k(Q1Q2/r)
How many steps are there in the formation of an ionic solid?
5
What is each step in the formation of an ionic solid, their equation and are they endo v. exothermic?
1.Sublimation of the solid metal.
M(s) → M(g) [endothermic]
2.Ionization of the metal atoms.
M(g) → M+(g) + e− [endothermic]
3.Dissociation of the nonmetal.
1/2X2(g) → X(g) [endothermic]
4.Formation of X− ions in the gas phase.
X(g) + e− → X−(g) [exothermic]
5.Formation of the solid MX.
M+(g) + X−(g) → MX(s)
[quite exothermic]
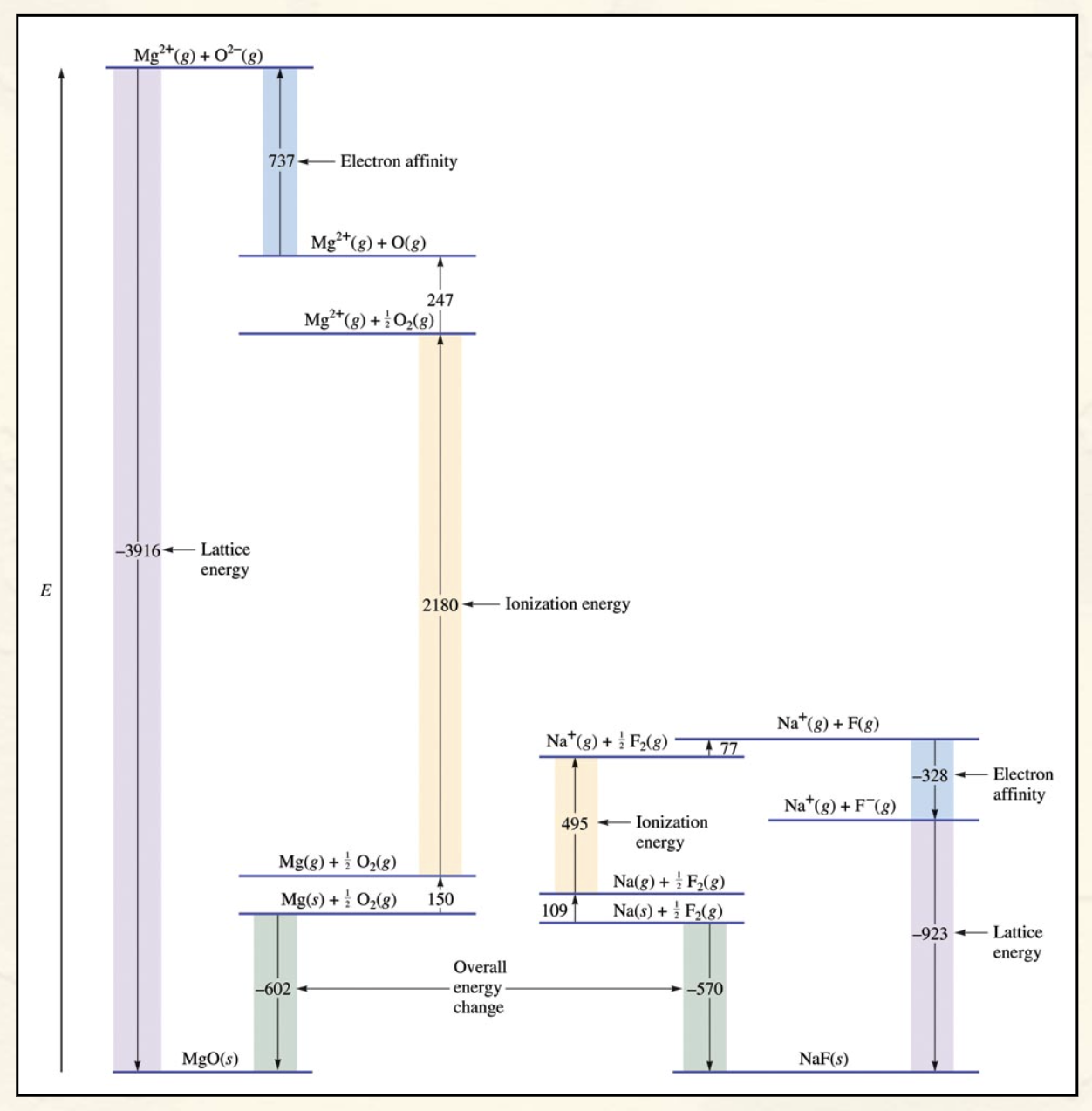
Comparing Energy Changes Picture
A graph of the formation of an ionic solid
Each step either requires or produces energy, the sum of all these values is the energy let out when an ionic solid is made.
The Final steps where the ions become a solid releases the amount of energy related to its lattice energy.
Overall the starting point is higher in energy then the final equation, which therefore shows its an exothermic reaction
Partial Ionic Character of Covalent Bonds
None of the bonds reaches 100% ionic character even with compounds that have a large electronegativity difference (when tested in the gas phase).
%ionic character of a bond = (measured dipole moment of X - Y / calculated dipole moment of X+Y-) x 100
CONCEPTUALLY: The fluorine has more attraction for an electron than does lithium. Both have valence electrons in the same principal energy level (the 2nd), but fluorine has a greater number of protons in the nucleus. Electrons are more attracted to a larger nucleus (if the principal energy level is the same).
The fluorine also has more attraction for an electron than does iodine. In this case the nuclear charge of iodine is greater, but the valence electrons are at a much higher principal energy level (and the inner electrons shield the outer electrons).
The greater the electronegativity difference..
the higher percent ionic character
Models are…
attempts to explain how nature operates on the microscopic level based on experiences in the macroscopic world.
The 5 fundamental properties of models are..
A model does not equal reality.
Models are oversimplifications, and are therefore often wrong.
Models become more complicated and are modified as they age.
We must understand the underlying assumptions in a model so that we don’t misuse it.
When a model is wrong, we often learn much more than when it is right.
Bond Energies:
To break bonds, energy must be added to the system (endothermic).
To form bonds, energy is released (exothermic).
Reactants - Products
The Localized electron model is a model…
composed of atoms that are bound together by sharing pairs of electrons using the atomic orbitals of the bound atoms.
Electron pairs are assumed to be localized on a particular atom or in the space between two atoms:
Lone pairs – pairs of electrons localized on an atom
Bonding pairs – pairs of electrons found in the space between the atoms
Steps to determine the localized electron model:
Description of valence electron arrangement (Lewis structure).
Prediction of geometry (VSEPR model).
Description of atomic orbital types used to share electrons or hold lone pairs.
A Lewis Structure shows… and reflects…
Shows how valence electrons are arranged among atoms in a molecule.
Reflects central idea that stability of a compound relates to noble gas electron configuration.
The Duet Rule is the idea that..
Hydrogen forms stable molecules where it shares two electrons
The Octet rule is the idea that…
Elements form stable molecules when surrounded by eight electrons
A Single Covalent Bond is a…
covalent bond in which two atoms share one pair of electrons.
H-H
A Double Covalent Bond is a bond..
in which two atoms share two pairs of electrons.
O=C=O
A Triple Covalent Bond is a…
covalent bond in which two atoms share three pairs of electrons.
N≡N
3 steps for writing a Lewis Structure:
Sum the valence electrons from all the atoms.
Use a pair of electrons to form a bond between each pair of bound atoms.
Atoms usually have noble gas configurations. Arrange the remaining electrons to satisfy the octet rule (or duet rule for hydrogen).
Boron tends to form compounds in which the boron atom..
has fewer than eight electrons around it (does not have a complete octet).
When can you exceed the octect rule if necessary?
When it is necessary to exceed the octet rule for one of several third-row (or higher) elements, place the extra electrons on the central atom.
4 details about the octet rule:
C, N, O, and F should always be assumed to obey the octet rule.
B and Be often have fewer than 8 electrons around them in their compounds.
Second-row elements never exceed the octet rule.
Third-row and heavier elements often satisfy the octet rule but can exceed the octet rule by using their empty valence d orbitals.
Can more than one valid/equal Lewis Structure be written for a particular molecule?
Yes! Look at NO3-
The Actual structure is an average of the…
resonance structures
Why is resonance possible?
Electrons are really delocalized – they can move around the entire molecule, which is why the actual structure is an average.
Formal Charge Overview:
Used to evaluate nonequivalent Lewis structures.
Atoms in molecules try to achieve formal charges as close to zero as possible.
Any negative formal charges are expected to reside on the most electronegative atoms.
The sum of the formal charges of all atoms in a given molecule or ion must equal the overall charge on that species.
How do you calculate formal charge?
#of valence electrons - # of valence electrons assigned to the atom in the molecule
ASSUME: Lone pair electrons belong entirely to the atom in question, and shared electrons are divided EQUALLY between the two sharing atoms.
RULE: the sum of the formal charges must equal the overall charge on that species.
2 Biggest rules governing formal charge:
Formal charges closest to zero and with any negative formal charges on the most electronegative atoms are considered the best to describe the bonding in the molecule or ion.
The 3 biggest formal charge examples:
SO4-2
SO3
BrO3-
Bond order is
theh number of chemical bonds between a pair of atoms.
ex. F2 is 1, O2 is 2, and N2 is 3.
In molecules with two or more resonance forms the bond order does not have to be an integer. How do you determine the bond order?
Essentially the number of sticks - the amount of domains.
Count the number of bonds in the molecule, then divide that number but the amount of bonds it could have.
For SO3:
Count that there is 4 bonds made (one double and two single)
Then count that one O to S bond could be a double bond, single bond, or another single bond. It has 3 different options to bond.
4/3 = 1.33 which is the bond length.
SHOULD BE GREATER THAN ONE
What is the VSEPR Model:
Valence Shell Electron-Pair Repulsion.
The structure around a given atom is determined principally by minimizing electron pair repulsions.
4 steps to apply the VSEPR Model:
Draw the Lewis structure for the molecule.
Count the electron pairs and arrange them in the way that minimizes repulsion (put the pairs as far apart as possible).
Determine the positions of the atoms from the way electron pairs are shared (how electrons are shared between the central atom and surrounding atoms).
Determine the name of the molecular structure from positions of the atoms.
If a molecule has polar bonds will it always be polar?
NO, a good example is CO2
It contains polar bonds since its electronegativity difference is between 0.5 and 1.9, but overall it is non polar since it is symmetrical.
True or false:
Lone pairs make a molecule polar.
FALSE:
Lone pairs often contribute to molecular polarity by distorting geometry, but they don't guarantee it, as symmetric arrangements (like XeF₄) with lone pairs can still be nonpolar since they cancel out
What determines bond polarity versus molecular polarity?
Bond polarity is determined by the electronegativity difference (END), 0.5 and 1.9 threshold numbers
Molecular polarity is determined by symmetry and dipoles
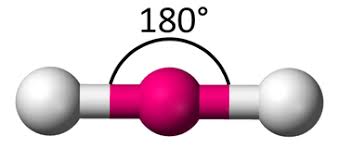
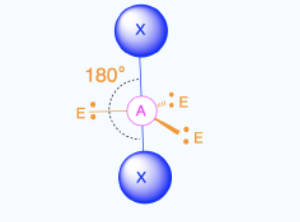
Linear (1)
Bond Angles: 180
Number of electron pairs (both bond and lone pairs): 2
Number of lone pairs: 0
Hybridization: sp (2 domains)
Electronic Geometry: Linear
Trigonal Planar
Bond Angles: 120
Number of electron pairs (both bond and lone pairs): 3
Number of lone pairs:0
Hybridization: sp2
Electronic Geometry: Trigonal Planar
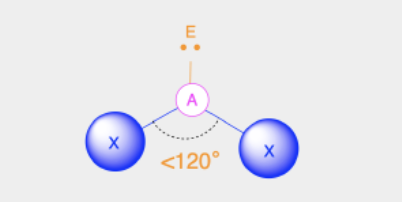
Bent
Bond Angles: 119
Number of electron pairs (both bond and lone pairs): 3
Number of lone pairs: 1
Hybridization: sp2
Electronic Geometry: Trigonal Planar
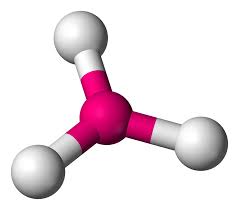
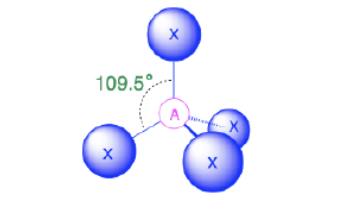
Tetrahedral
Bond Angles: 109.5
Number of electron pairs (both bond and lone pairs):4
Number of lone pairs:0
Hybridization: sp3
Electronic Geometry: tetrahedral
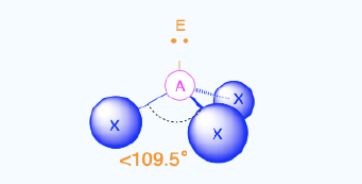
Pyramidal
Bond Angles:107.5
Number of electron pairs (both bond and lone pairs): 4
Number of lone pairs: 1
Hybridization: sp3
Electronic Geometry: tetrahedral
Bent
Bond Angles: 105.5
Number of electron pairs (both bond and lone pairs): 4
Number of lone pairs: 2
Hybridization: sp3
Electronic Geometry: tetrahedral

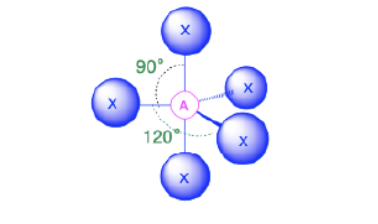
Trigonal Bipyramidal
Bond Angles: 120; 90
Number of electron pairs (both bond and lone pairs): 5
Number of lone pairs: 0
Hybridization: sp3d
Electronic Geometry:Trigonal Bypyramidal
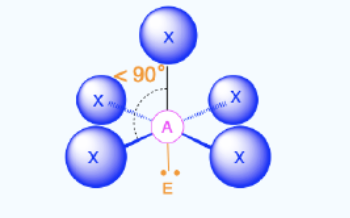
See-saw
Bond Angles: 119; 90
Number of electron pairs (both bond and lone pairs): 5
Number of lone pairs: 1
Hybridization: sp3d
Electronic Geometry: Trigonal Bypyramidal
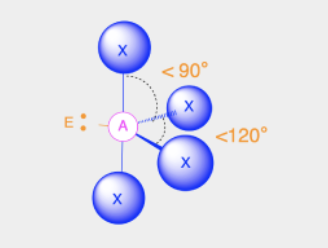
T-Shaped
Bond Angles: 90
Number of electron pairs (both bond and lone pairs): 5
Number of lone pairs: 2
Hybridization: sp3d
Electronic Geometry: Trigonal Bypyramidal
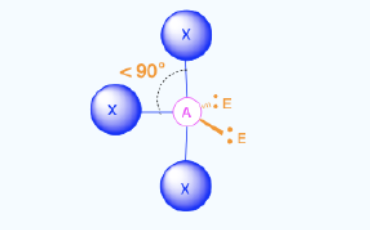
Linear (2)
Bond Angles: 180
Number of electron pairs (both bond and lone pairs): 5
Number of lone pairs: 3
Hybridization: sp3d
Electronic Geometry: Trigonal Bypyramidal
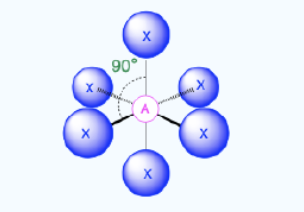
Octahedral
Bond Angles: 90
Number of electron pairs (both bond and lone pairs): 6
Number of lone pairs: 0
Hybridization: sp3d2
Electronic Geometry: Octahedral
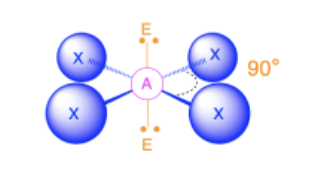
Square Pyramidal
Bond Angles: 90
Number of electron pairs (both bond and lone pairs): 6
Number of lone pairs: 1
Hybridization: sp3d2
Electronic Geometry: Octahedral
Square Planar
Bond Angles: 90
Number of electron pairs (both bond and lone pairs): 6
Number of lone pairs: 2
Hybridization: sp3d2
Electronic Geometry: Octahedral
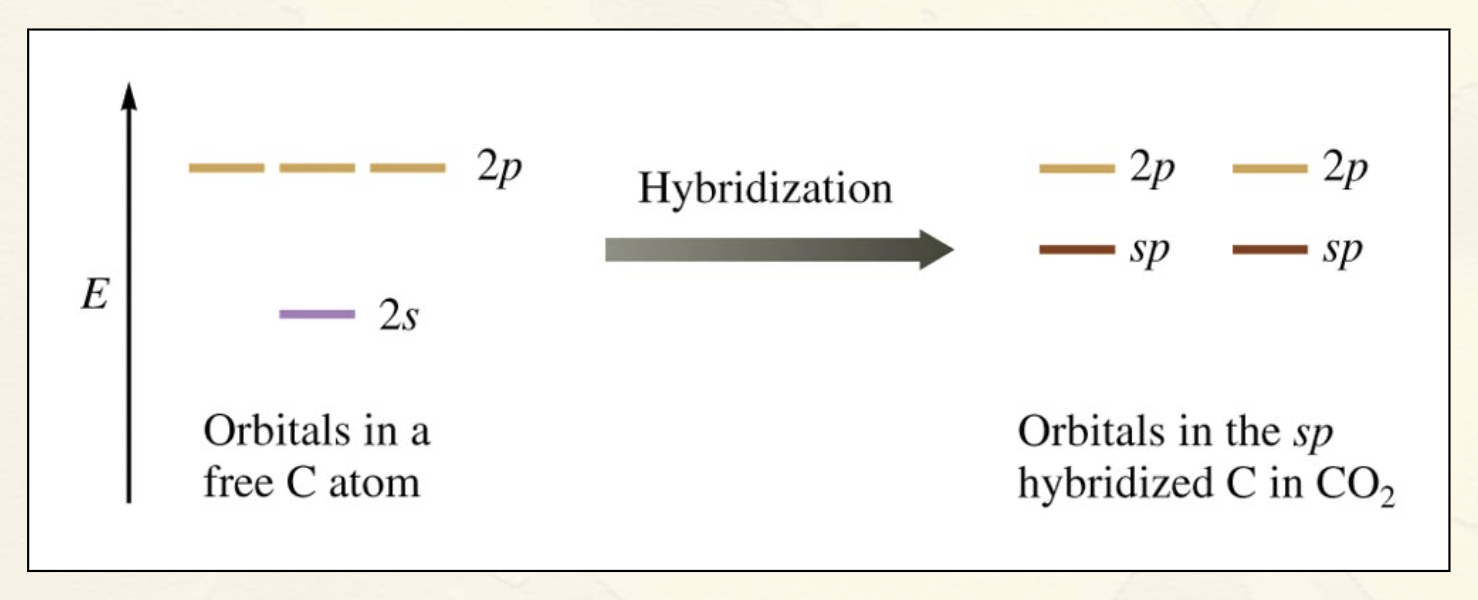
What is hybridization?
The mixing of the native atomic orbitals to form special orbitals for bonding.
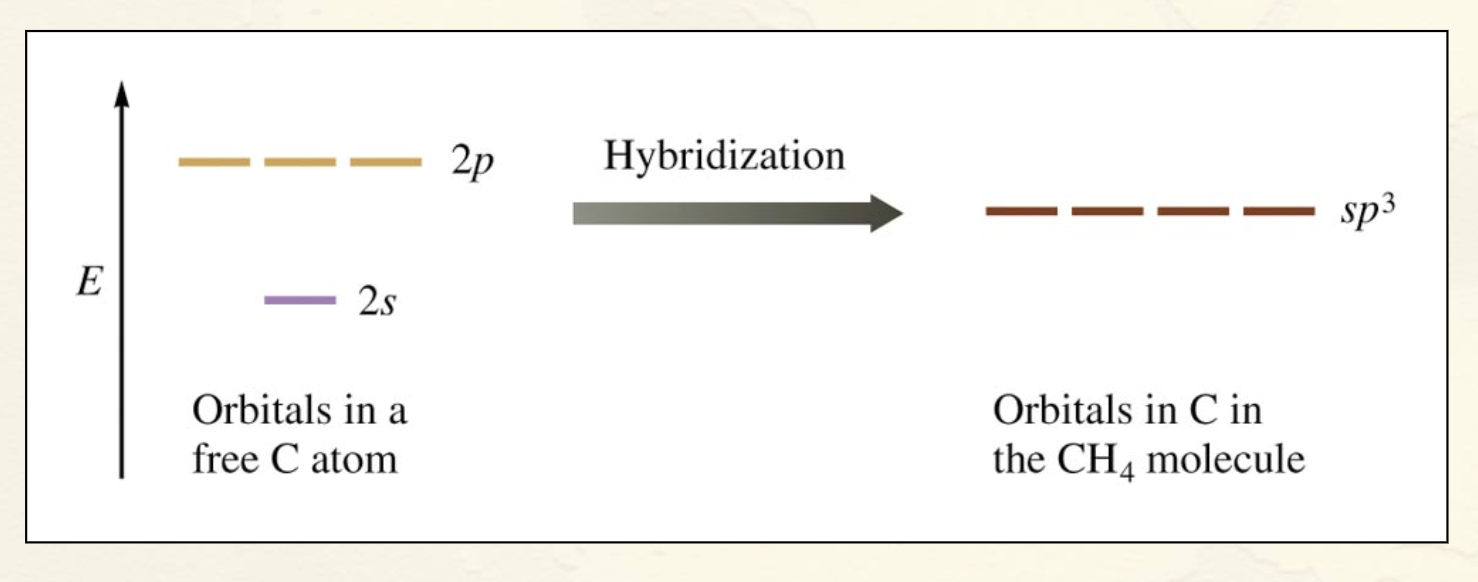
sp3 Hybridization:
Combination of one s and three p orbitals
Whenever a set of quivalent tetrahedral atomic orbitals is required by an atom, the localized electron model assumes that the atom adopts a set of sp3 orbitals; the atom becomes sp3 hybridized
How would you draw the before and after?
3 2p orbitals and 1 2s orbital come together to become 4 sp3 orbitals.
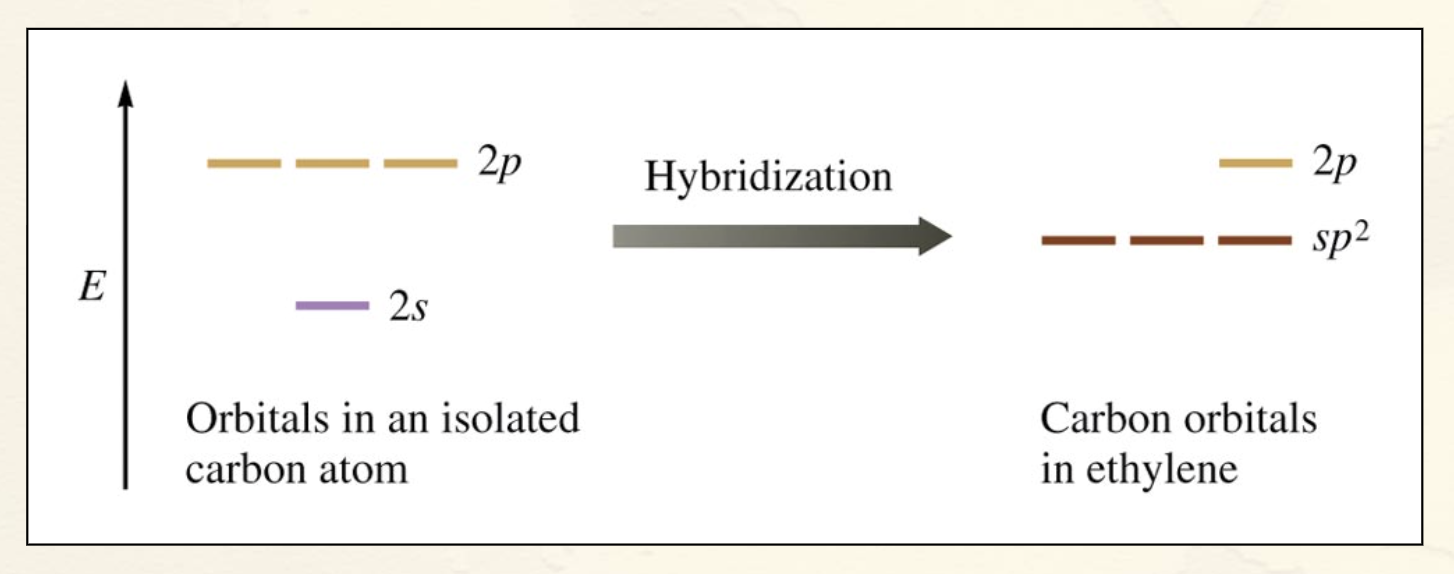
sp2 Hybridization:
Combination of one s and two p orbitals.
Gives a trigonal planar arrangement of atomic orbitals.
One p orbital is not used.
Oriented perpendicular to the plane of the sp2 orbitals.
Sigma (σ) Bond
Electron pair is shared in an area centered on a line running between the atoms.
Sigma bonds are free to rotate
Pi (π) Bond
Forms double and triple bonds by sharing electron pair(s) in the space above and below the σ bond (double and triple bonds).
Uses the unhybridized p orbitals.
Pi bonds prevent rotation
Whats an Orbital Energy-Level Diagram for sp2 Hybridization (picture)?
sp Hybridization + picture:
Combination of one s and one p orbital.
Gives a linear arrangement of atomic orbitals.
Two p orbitals are not used.
Needed to form the π bonds
What is delocalization?
Describes molecules that require resonance.
In molecules that require resonance, it is the π bonding that is most clearly delocalized, the σ bonds are localized.
p orbitals perpendicular to the plane of the molecule are used to form π molecular orbitals.
The electrons in the π molecular orbitals are delocalized above and below the plane of the molecule.
Draw the lewis structure for HCN. Which hybrid orbitals are used and how many sigma and pi bonds are there?
sp hybrid orbitals are used
two sigma bonds and two pi bonds
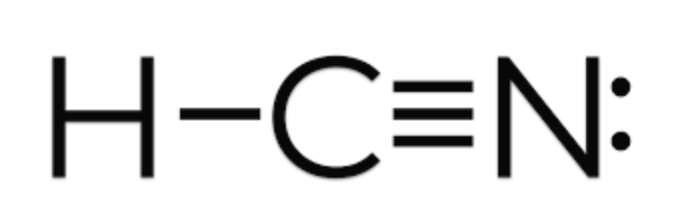
The greater a substance’s IMFs…
the greater its melting point and boiling point.
Hydrogen bonds can only occur between…
H-F H-N or H-O Bonds
Drawn in with dashed lines
Hydrogen bonding is when
H is bonded to O, N, or F and is attracted to a lone pair on a highly electronegative neighboring atom
Hydrogen bonding is about 5x the strength of
a dipole-dipole IMF
Stronger intermolecular forces =
higher melting and boiling points
Actual bonds (ionic, covalent, metallic, network covalent) …
are much stronger than IMFs
Intra vs Inter Strength:
Inter (intermolecular forces) are less strong than intra (ionic, metallic, covalent forces)
Hierarchy Strongest to Weakest Forces:
1. Network Covalent
2. Ionic/Metallic
3. Molecular Covalent
i. ion-dipole
ii. hydrogen bonding
iii. dipole-dipole
iv. LDF
What to look for:
Nonpolar (symmetric) molecules (N2,O2,H2, Cl2, F2, CH4) ⇒ London forces
Molecules with H-N, H-O, H-F ⇒ hydrogen bonding
Other polar molecules ⇒ dipole forces
High melting point and boiling point = strong force of attraction between molecules.
What is an IMF?
InterMolecular Force i.e. force of attraction between molecules (NOT a bond).
A substance is a gas and another is a liquid at 298 K. Which has weaker IMFs?
The gas has weaker IMF’s
What are ion-dipole attractions?
Attraction between molecules of water and ions dissolved in it.
What force of attraction applies to nonpolar molecules (and noble gases)?
London Dispersion Forces
Properties of Ionic Compounds:
Very high melting/boiling point
has conductivity in liquid and aqueous forms
brittle
low luster crystals
forces between particles: electrostatic attractions
Properties of Metallic Compounds:
High melting/boiling point
good conductor
is malleable
high luster (shiny)
forces between particles: metallic bonds
Properties of Molecular Covalent Compounds
low to moderately high melting/boiling point
poor conductor
not very malleable
dull, low luster
forces between particles: LDFs, dipole-dipole forces, hydrogen bonds
Properties of Network Covalent Compounds:
very high melting/boiling point
poor thermal and electrical conductor
very hard
most common examples: Diamond, quartz, SiO2
forces between particles: Covalent Bonds
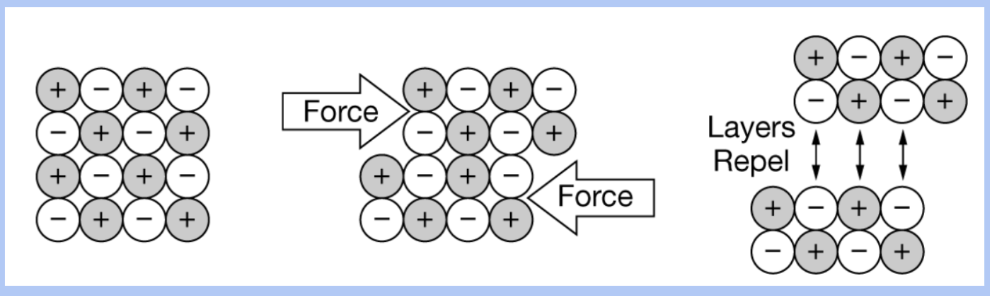
Why are ionic compounds brittle?
a strong repulsive force is created when anions or cations within the lattice align
Why are ionic compounds good conductors in aqueous solutions?
When they dissolve, they dissociate 100%, therefore their rigid crystal lattice structure breaks and frees positive and negative ions.
These ions become surrounded by water molecules and then are free to move which allows them to carry electrical change through the solution.
More bonds =
shorter bond length
ex. a triple bond is shorter than a single bond