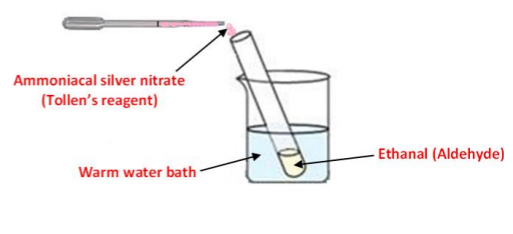
To show the reaction between ethanol and ammoniacal silver nitrate /Tollen's reagent (silver mirror test)
1/6
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
7 Terms
Procedure
➢ The ethanal is placed in a test tube in a warm water bath
➢ Using a dropper, ammoniacal silver nitrate is added to the ethanal
Result: The colourless Ag+ solution reacts and a silver mirror is formed on the inside of the test tube
Note: When repeated with propanone in place of ethanal, no colour change is observed
By what other name is ammoniacal silver nitrate known?
Tollen’s reagent
Why must Tollen’s reagent always be freshly made up?
If Tollen’s reagent is stored, it is likely explosive products could form
Write the half reactions that occur when ammoniacal silver nitrate reagent is added to ethanal
Ag+ → Ag
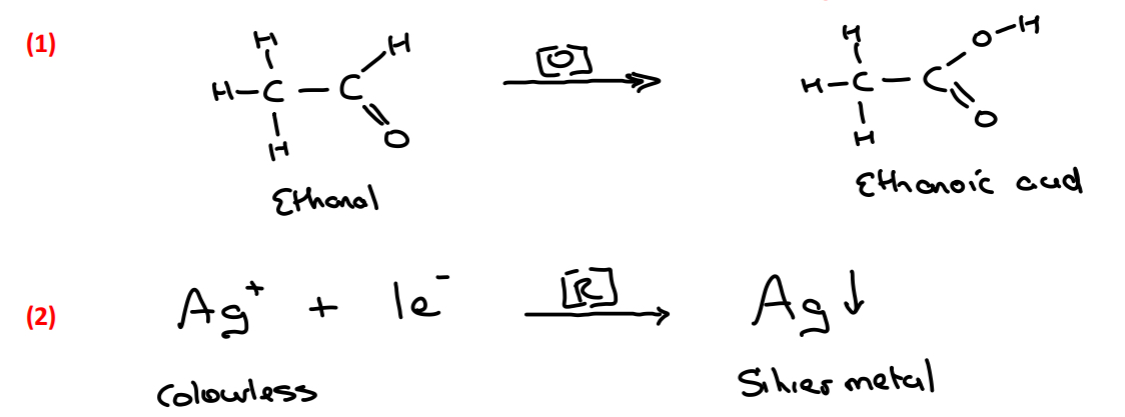
Explain the colour change that occurs when ammoniacal silver nitrate is added to ethanal
• Tollen’s reagent contains Ag+ ions causing a colourless solution
• When reacted with ethanal Ag+ ions are reduced (gain 1 e-) to form Ag (silver)
• Ag precipitates out of solution causing a silver mirror
In each test performed, no change is observed if ethanal is replaced with propanone. Explain.
• Propanone is a ketone
• Ketones are not easily oxidised
• A very strong oxidising agent would be required
Identify the organic substance produced in all 3 tests
Ethanoic acid