5.2.2 Enthalpy and entropy
1/29
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
30 Terms
what is entropy
measure of dispersal of energy in a system
what does the greater the entropy mean
the more disordered the system
symbol for entropy
S
symbol for entropy change
ΔS
what happens to entropy as you go solid to liwuid to gas
becomes more disordered
what happens to entropy as you dissolve a solid
increases its entropy
what happens to entropy as there are more gaseous particles
increases
units of entropy change
JK^-1mol^-1
when will chemical reactons only take place with entropy change
when overall entropy change is positive
entropy change of system equation
ΣSproducts - ΣSreactants
entropy change of surroundings equation
-enthalpy change/temperature
what is units of enthalpy change in entropy change of surroundings equation
Joules
what is units of temperature in entropy change of surroundings equation
K
total entropy change
entropy change of system + entropy change of surroundings
what happens to entropy as temperature increases
entropy increases
what is free energy change
measure used to predict whether a reaction is feasible
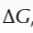
feasible reaction
reaction that once started will carry on to completion without any energy being supplied to it
when is a reaction feasbile
if free energy change is negative or zero
gibbs / free energy change equation
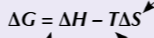
units of free energy change
Jmol^-1
units of enthalpy change in gibbs equation
Jmol^-1
why might a reaction not take place even if △G is negative
high activation energy,slow rate of reaction
equation to work out temperature at which reaction is feasible
△H/△S
id a reaction is exothermic and has a positive entropy change what will △G always be
negative
id a reaction is endothermic and has a negative entropy change what will △G always be
positive
if a reaction is exothermic and has negative entropy change when will it be feasible
low temperature
if a reaction is endothermic and has positive entropy change when will it be feasible
high ttemperatres
gibbs equation in y=mx + c form
△G=-△s T + △H
what is the gradient of straigt line gibbs
-△S
what is the y intercept of straight lien gibbs
△H