Pharmaceutics Exam 1: Functional Groups and Stereochemistry
1/141
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
142 Terms
intermolecular force vs intramolecular force
inter: forces that exist between molecules
intra: forces that exist between atoms within molecules
strongest intermolecular force
ionic interactions
ionic interactions
exist between ions (salts/charged species)
strongest type of intermolecular force
ion-dipole forces
exist between an ion (charged species) and a dipole
hydrogen bonds
intermolecular attractions that exist between the hydrogen of one species and the oxygen/nitrogen/fluorine of another species
H-bonds are a type of dipole-dipole interaction (the strongest type), and are attractions not actual bonds
dipole-dipole interactions
attractions that exist between a partially positive portion of one species and a partially negative portion of another species
these are attractions and not actual bonds
van der Waals interactions
weak attractive forces between uncharged molecules
hydrophobic interactions (think "clumping") are the most commonly discussed
the number of intermolecular forces between molecules is ____ proportionally to the boiling point
directly
(increasing the number of forces increases the boiling point because more bonds must be broken, requiring more energy)
the molecular weight of a molecule is ____ proportionally to the boiling point
directly
electron-donating groups
substituents that "push" electron density toward a functional group to increase electron density --> more likely to accept a proton
ex. alkyl groups
electron-donating groups and acid/base character
decrease in acidity and increase in basicity
EDGS increase electron density, which increases the ability to accept a proton (ie. positively-charged proton is attracted to a more "negative" species)
electron-withdrawing groups
substituents that "pull" electron density away from a functional group to decrease electron density (disperse the cloud) --> more likely to donate a proton
ex. benzene, halogens, -SO2, -NO2
electron-withdrawing groups and acid/base character
increase in acidity and decrease in basicity
EWGs disperse electron density, making the species more likely to donate an electron ("floating" -H is less-attracted to the less-negatively charged species)
electropositivity
tendency to donate electrons (EDGs)
electronegativity
tendency to withdraw/disperse electrons (EWGs)
aromatic hydrocarbons
contain a benzene ring or a derivative of a benzene ring
aromatic hydrocarbons are ____ groups (in terms of electrons) because....
electron-withdrawing
they have a high electron density that results in a delocalization of electrons (they pull electrons toward themselves, making the R-group they are attached to more electropositive)
When aromatic hydrocarbons are metabolized, they are ____ by CYP45s to become more ____
hydroxylated
water-soluble
Why can aromatic hydrocarbons be carcinogenic?
the process of hydroxylating AHCs produces an epoxide intermediate, which experiences a lot of ring strain and is therefore highly reactive (toxic) to nucleophiles such as DNA
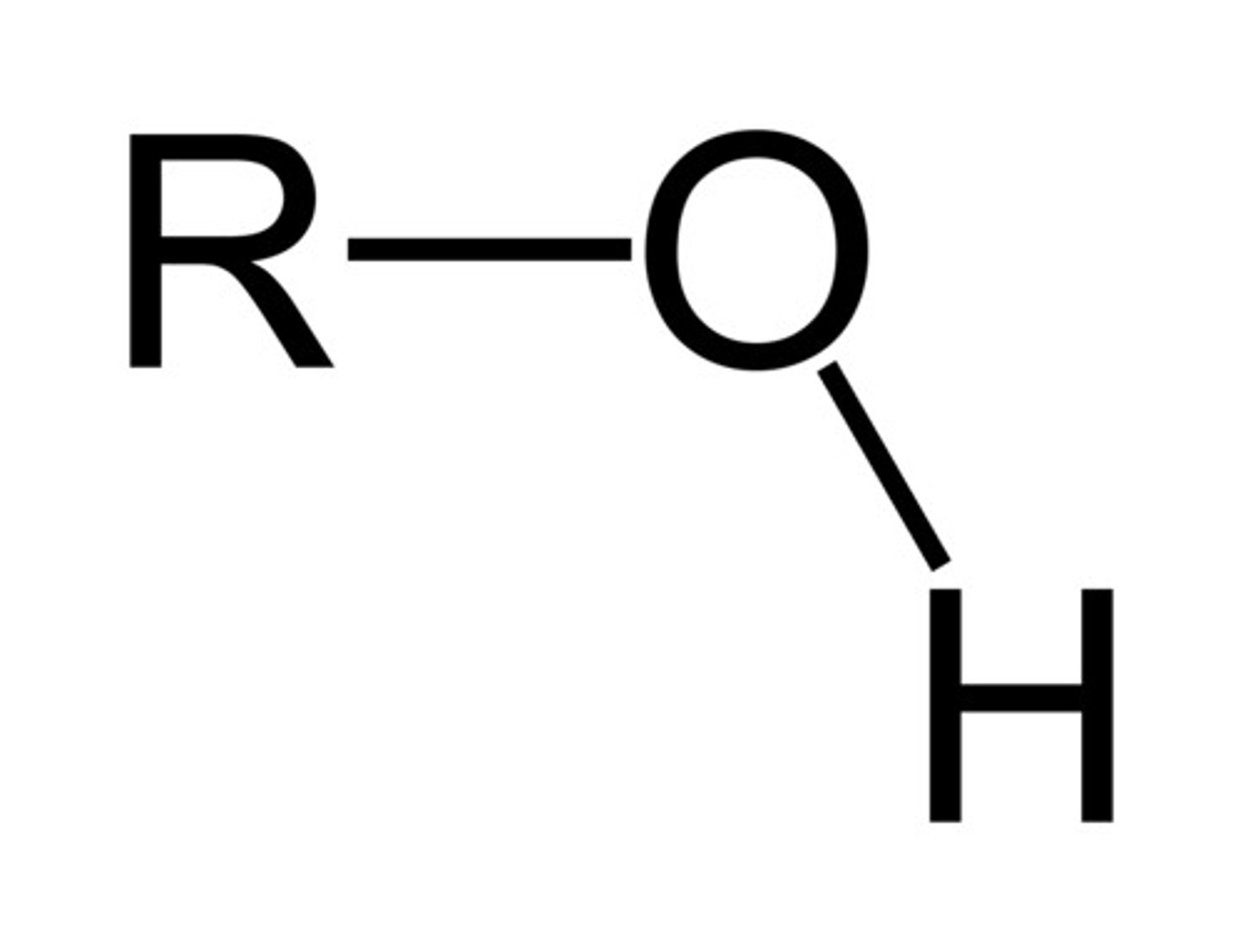
alcohols
organic compounds containing hydroxyl groups
in vitro, alcohols are susceptible to ____ reactions
oxidation
Why is methanol more toxic than ethanol?
oxidation of methanol by alcohol dehydrogenase produces formaldehyde (toxic), which is then metabolyzed to formic acid (also toxic)
oxidation of ethanol by ADH produces acetylaldehyde (toxic-ish), which is then metabolyzed to acetic acid (vinegar)
What is the treatment for methanol poisoning and why?
large amounts of ethanol, because ethanol competes with methanol for oxidation by alcohol dehydrogenase, preventing methanol from being converted to formaldehyde/formic acid
Why is 1-butanol more water-soluble than 2-butanol?
1-butanol experiences more van der Waals interactions than 2-butanol
the -OH group in the middle of 2-butanol disrupts vdWaals, which makes it less hydrophobic and therefore more water-soluble
phenols
characterized by an -OH attached to a benzene ring
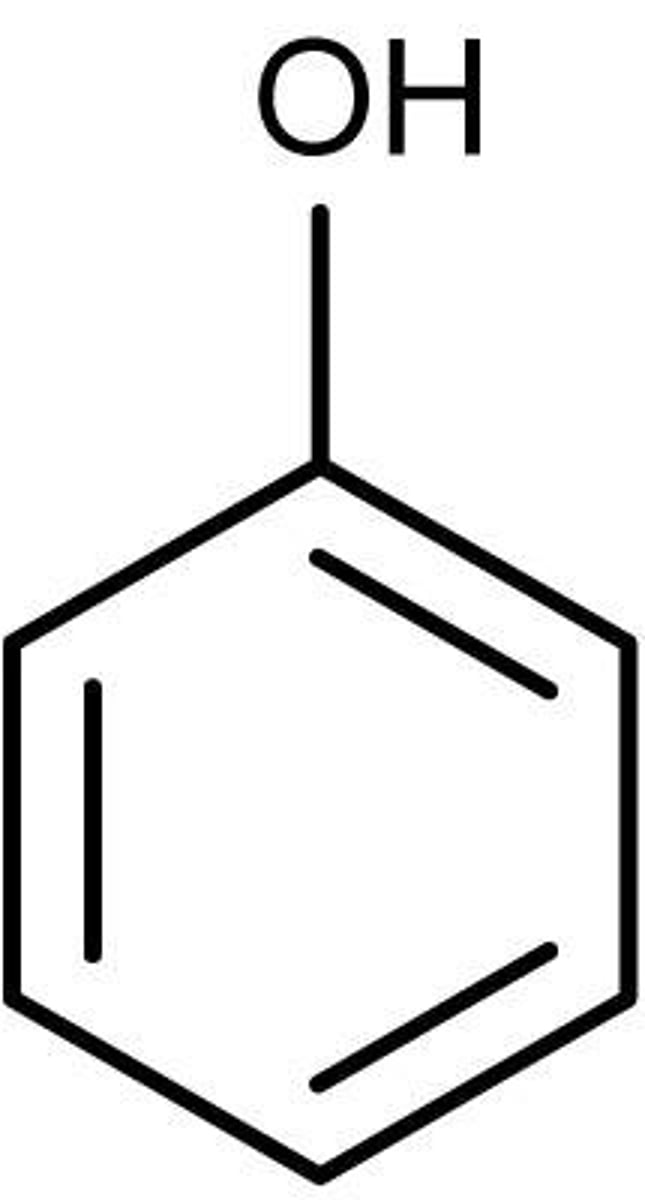
Are phenols acidic or basic and why?
weakly acidic
the negative charge on the oxygen after hydrogen donation is stabilized by resonance and the electron-delocalizing characteristic of aromatic hydrocarbons (electron-withdrawing character makes the O- charge of the conjugate acid less negative)
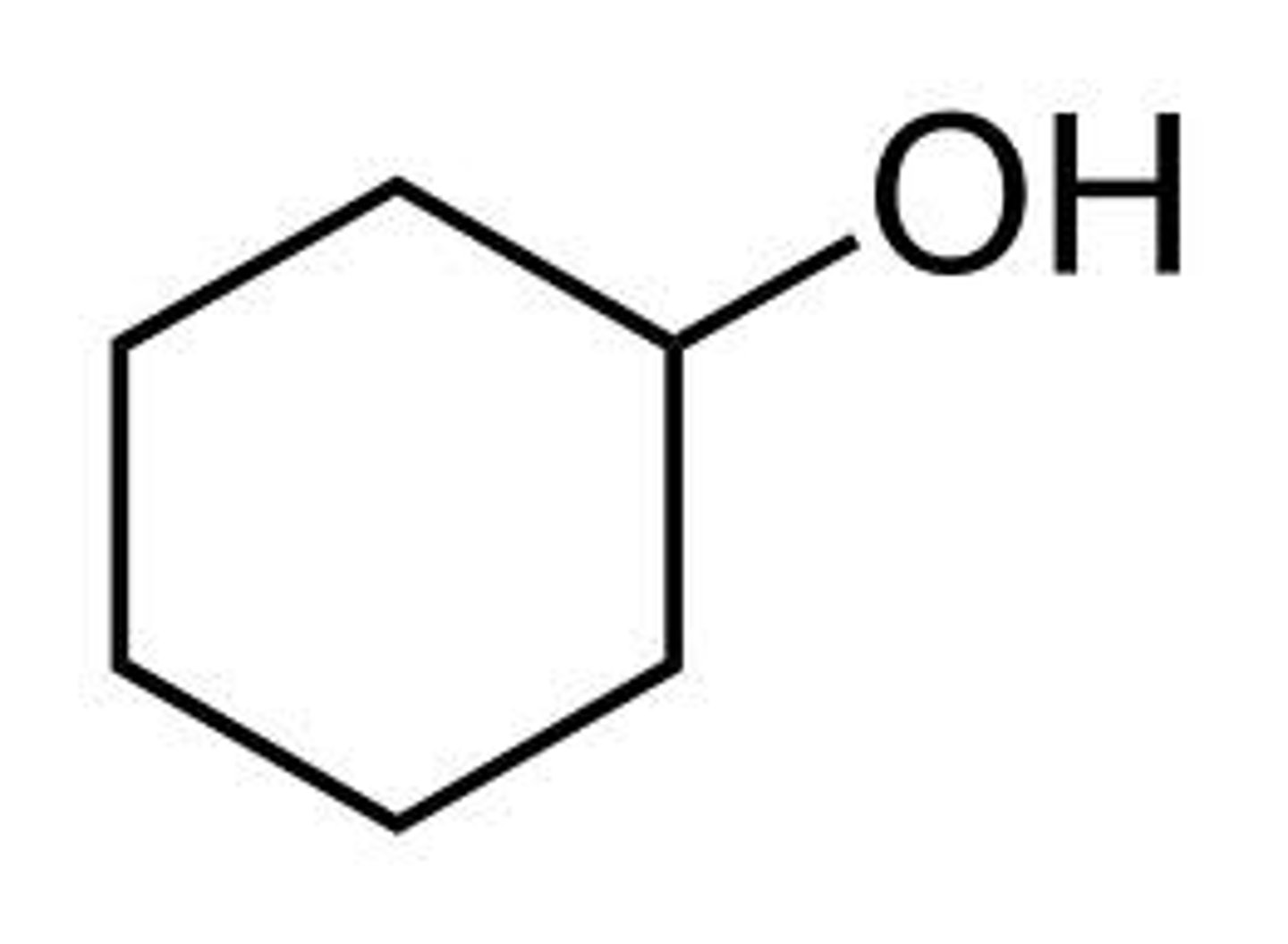
Why does phenol have a higher boiling point than cyclohexanol?
the constant conversion of cyclohexanol between chair and boat conformations results in less ability for hydrogen bond formation
phenols are readily ____ to guanine when exposed to air
oxidized
Vitamin E and phenols
Vitamin E is readily oxidized, so it acts as a shield (antioxidant) to prevent the oxidation of phenols
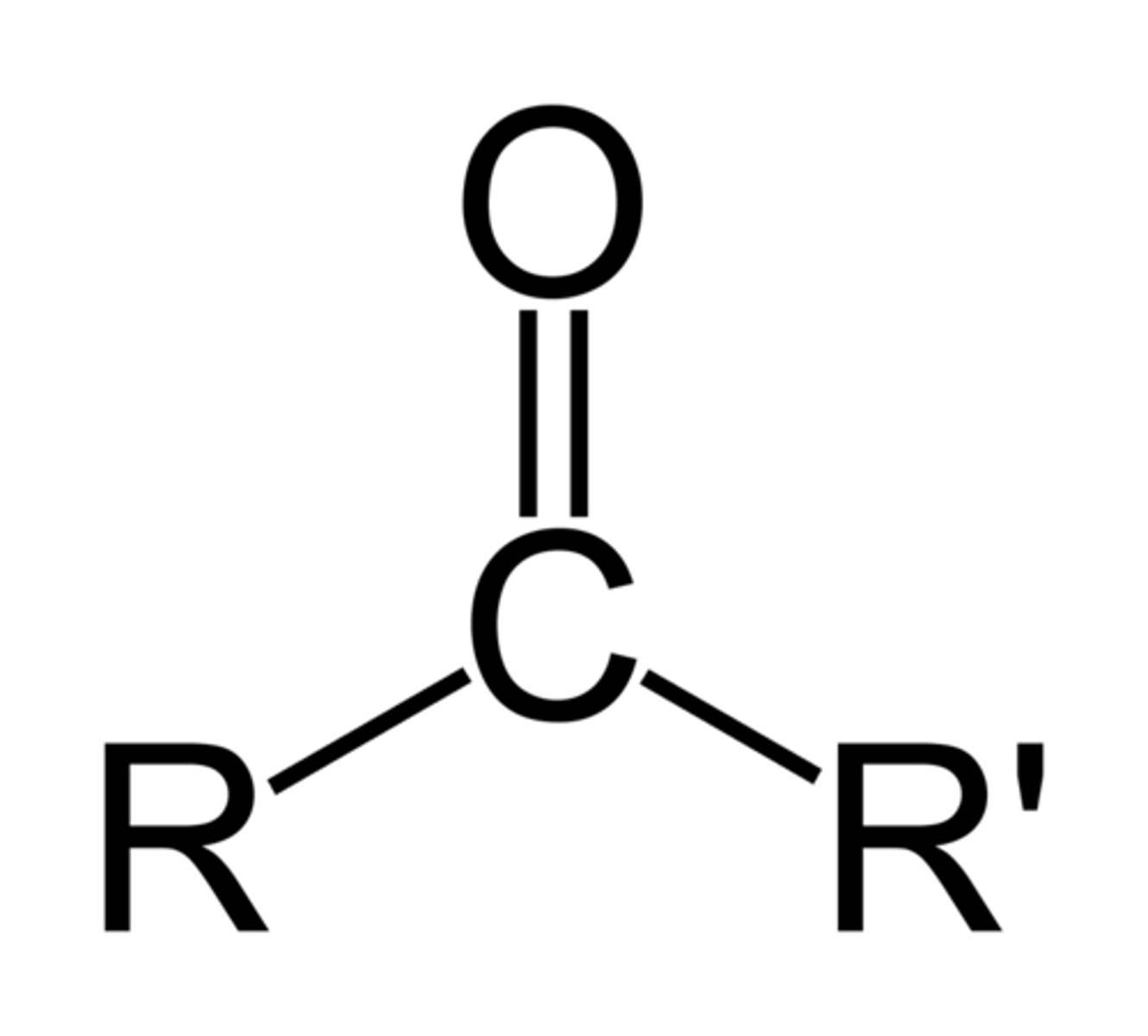
ketone
carbonyl group (C=O) is within carbon skeleton
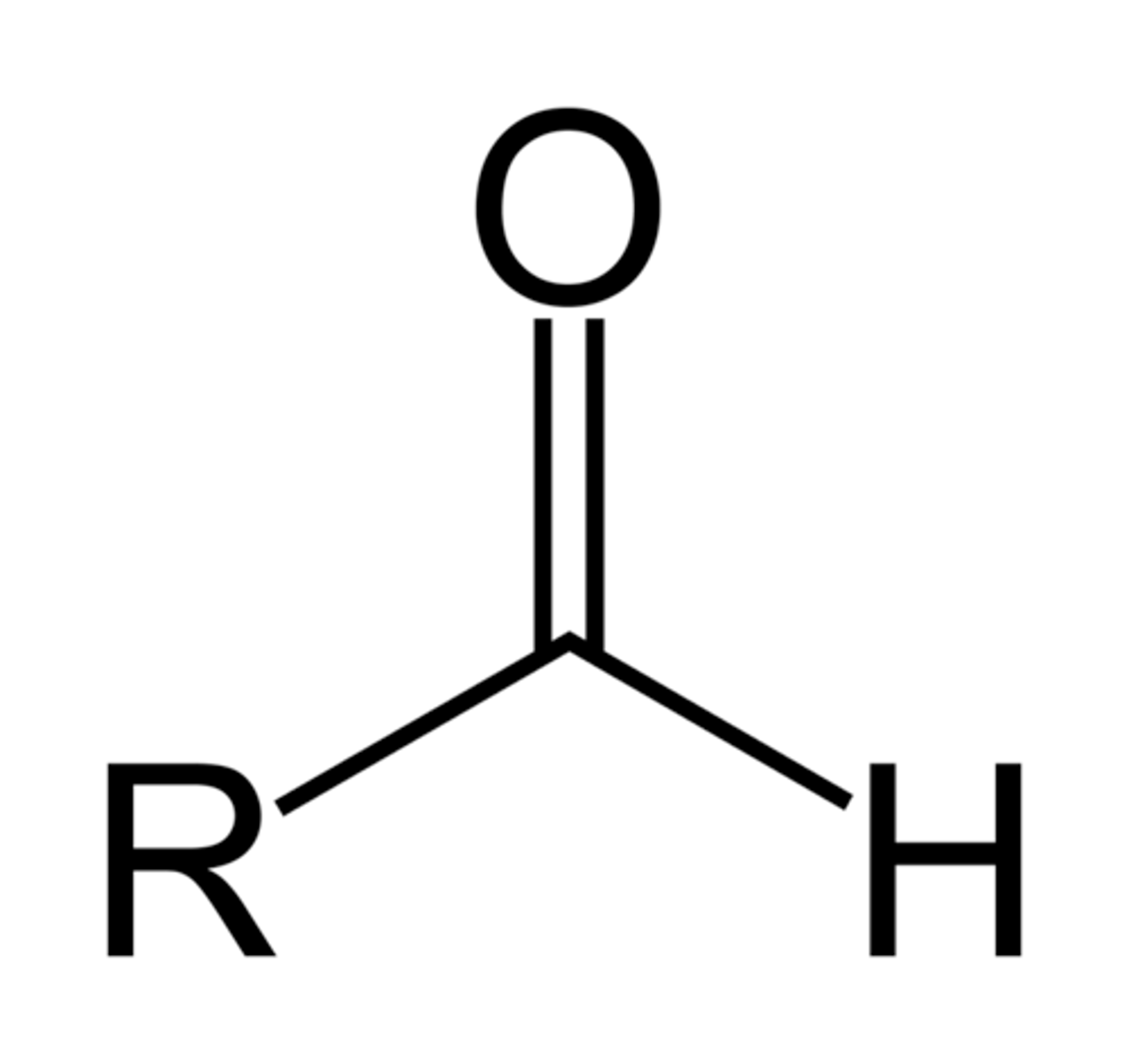
aldehydes
carbonyl group (C=O) is at the end of the carbon skeleton
Can ketones and aldehydes hydrogen bond?
they cannot hydrogen bond, but can dipole-dipole bond
tautomerization
conversion between a keto (left) and enol (right) form
a C=C bond is formed with the enol (ene + ol --> C=C + an -OH)
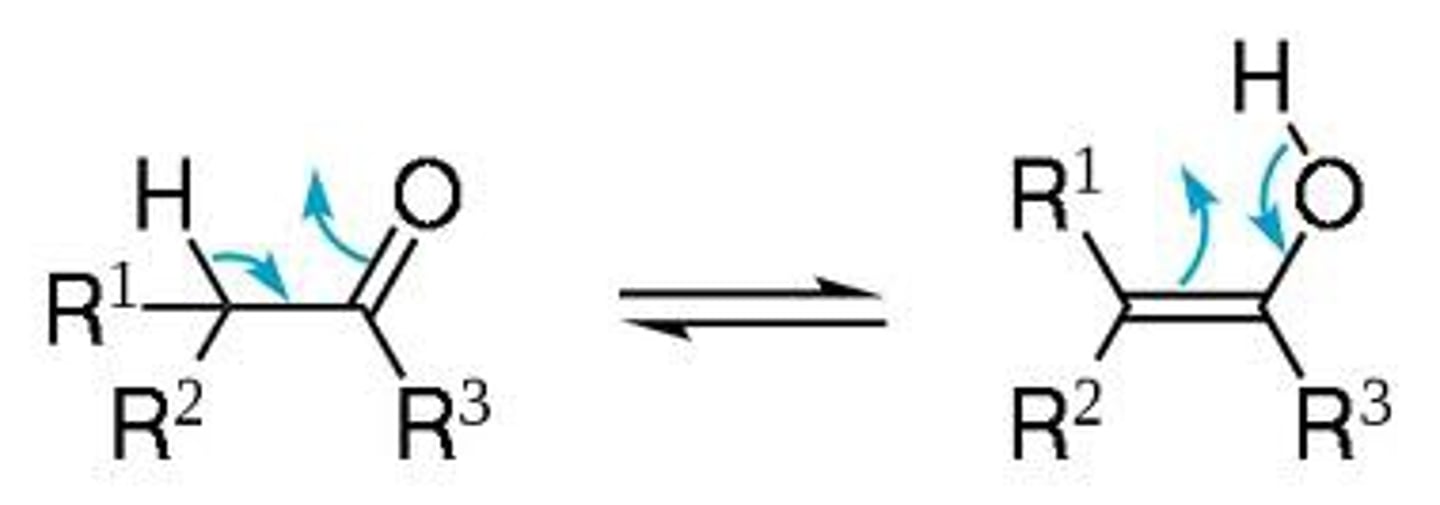
What is the affect of tautomerization in relation to acid-base character?
tautomerization makes the molecule more acidic
Increasing the length of the hydrocarbon chain of ketones and aldehydes results in a(n) ____ in boiling point and a(n) ____ in water solubility
increase
decrease
(more vdWaals possible)
Are ketones and aldehydes susceptible to air oxidation?
aldehydes are susceptible --> carboxylic acid
ketones are generally NOT because it is difficult to break a C-C bond (although they can be oxidized when attached to benzene)
hemiacetal
formed in acidic conditions when an alcohol is introduced to an aldehyde/ketone
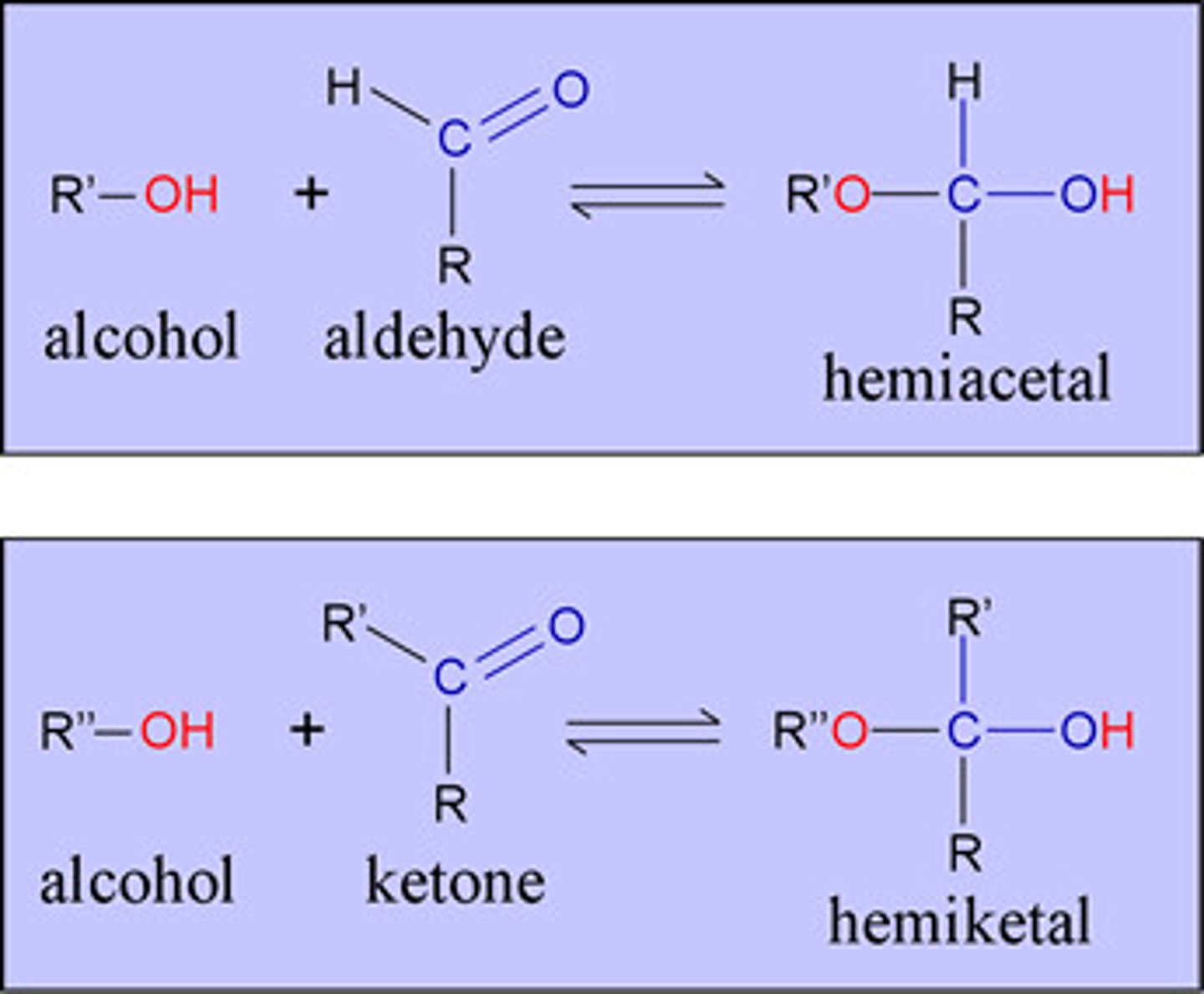
glucuronic conjugates are ____
aldehydes
The reason barbituric acid is approximately 3 pKa units more acidic than phenobarbital is due to:
A. The electron-withdrawing character of the benzene ring
B. The electron-donating character of the benzene ring
C. Its ability to tautomerize to form an enol
D. Its ability to accept a proton
The reason barbituric acid is approximately 3 pKa units more acidic than phenobarbital is due to:
A. The electron-withdrawing character of the benzene ring barbituric acid does not have a benzene ring
B. The electron-donating character of the benzene ring this would actually make barbituric acid more basic, if it even had a benzene ring
C. ITS ABILITY TO TAUTOMERIZE AND FORM AN ENOL
D. Its ability to accept a proton accepting a proton is a characteristic of bases
Are primary or tertiary alcohols more likely to be oxidized?
primary are more likely to be oxidized
tertiary alcohols are more stable
also, oxidation of alcohols results in the formation of a C=O bond and requires the C-H bond between the -OH-adjacent carbon to be broken, a bond which is not present in tertiary alcohols
amines
organic compounds with an amino group
"Vitamin" = life-giving amine
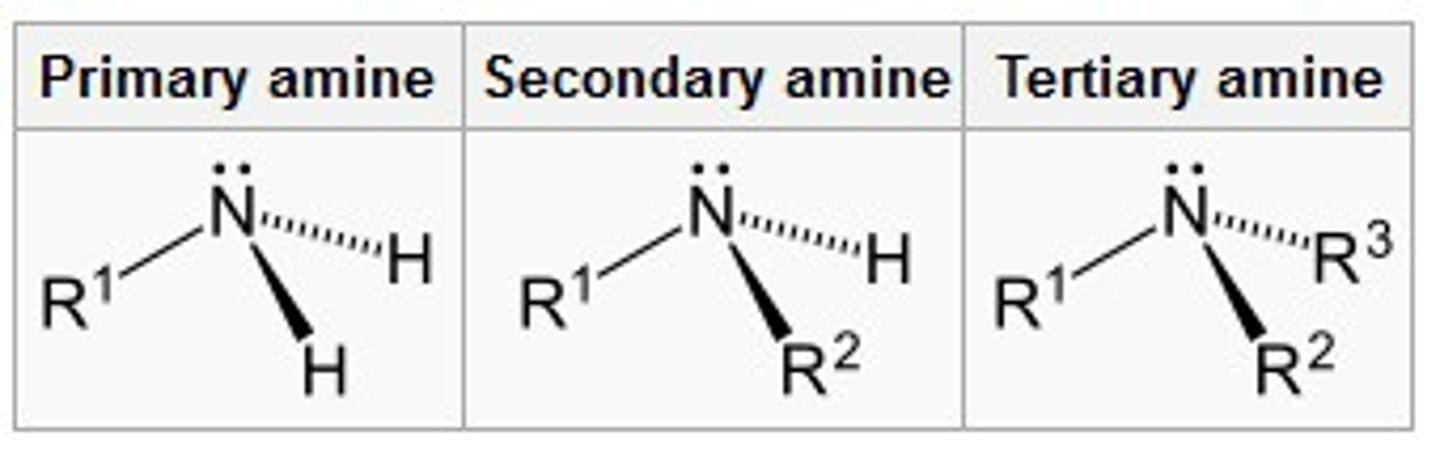
Are amines acidic or basic?
weakly basic
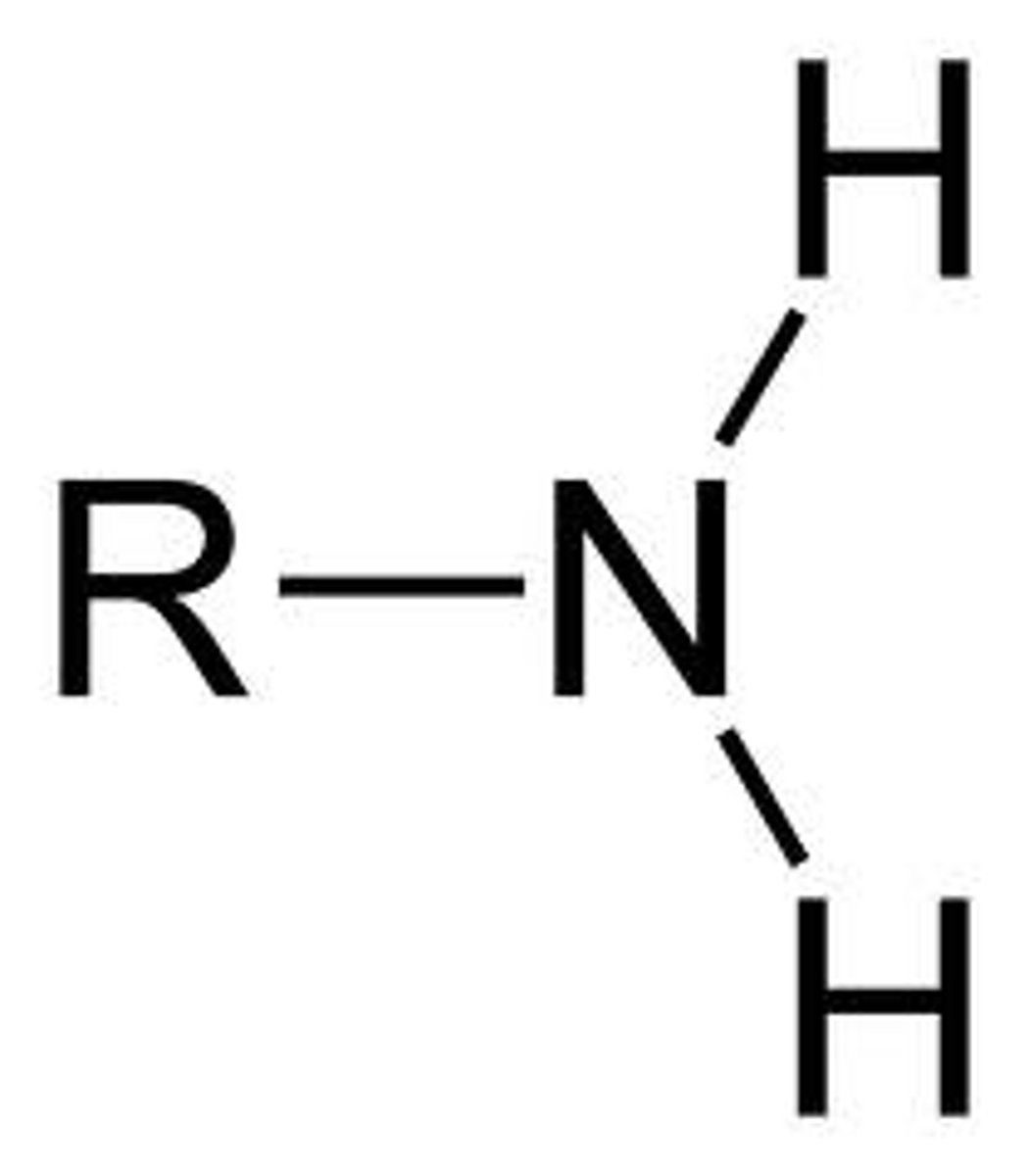
primary amine
an amine where the nitrogen atom is attached to one alkyl chain
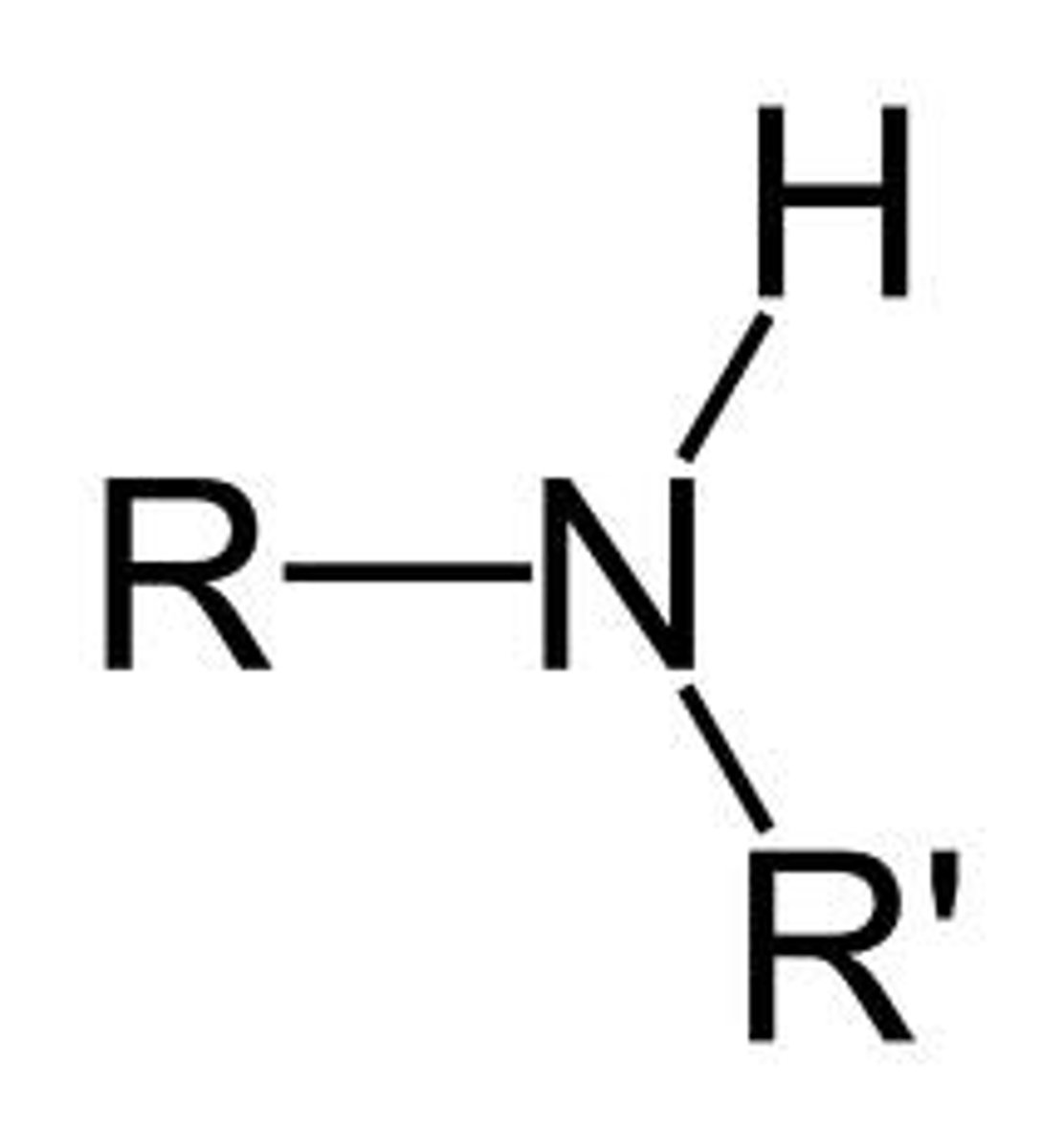
secondary amine
an amine where the nitrogen atom is attached to two alkyl chains
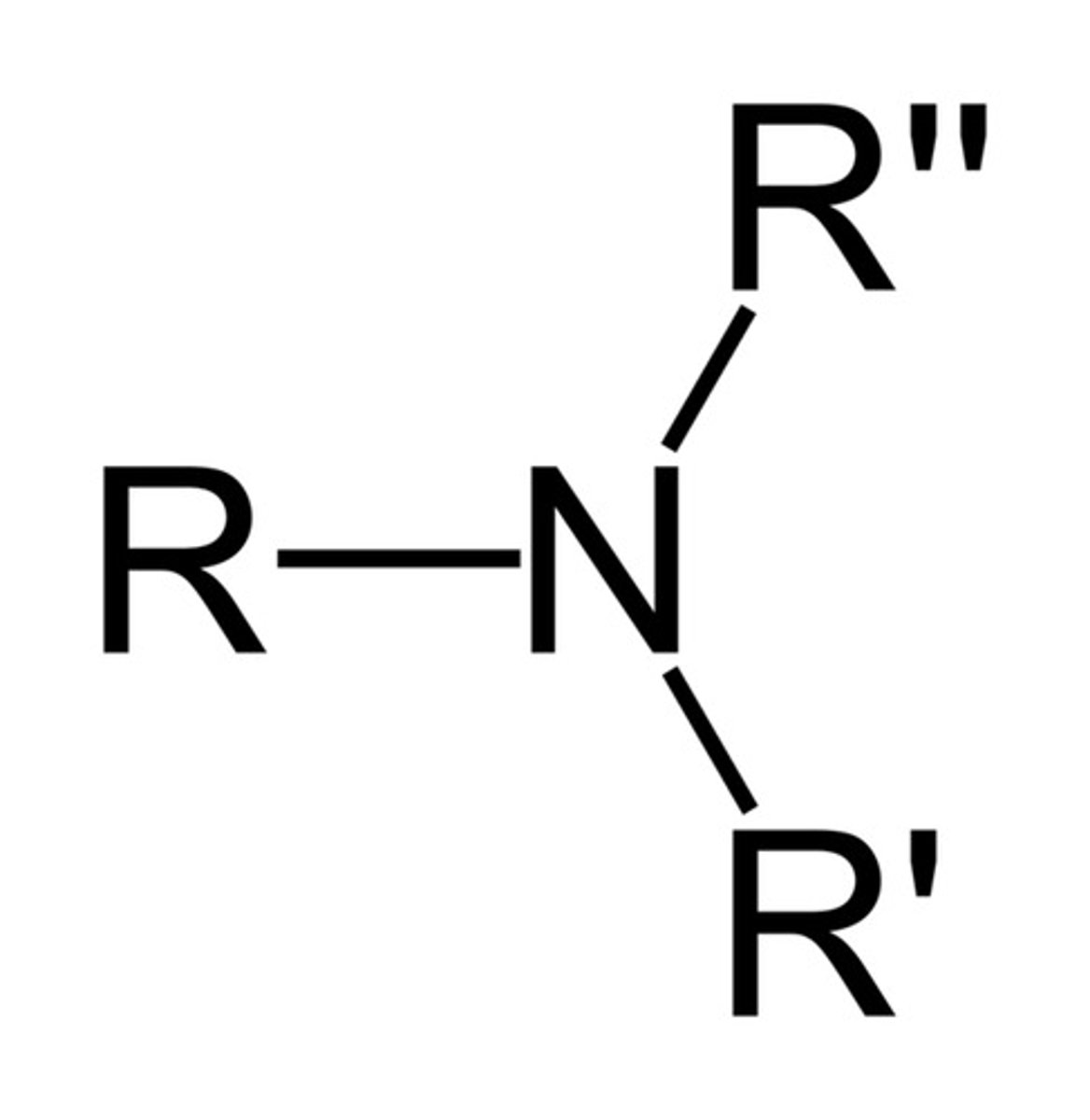
tertiary amine
an amine where the nitrogen atom is connected to 3 carbon atoms
quarternary amine
a configuration in which nitrogen has four bonds with other atoms --> positively-charged nitrogen
quarternary amines (ammonium salts) are unreactive/stable and "neutral"
rank the basicity of primary, secondary, and tertiary ethylamines
secondary > tertiary > primary
tertiary ethylamines experience more steric hindrance than secondary --> less basic
however, tertiary are more basic than primary because the ethyl groups "pump" electrons in towards the nitrogen, making it more likely to accept a proton than primary
rank the basicity of primary, secondary, and tertiary methylamines
secondary > primary > tertiary
secondary methylamines have more e-donating groups (-CH3) than primary amines --> makes the nitrogen more electronegative, and therefore more likely to accept a proton
tertiary methylamines experience steric hindrance, and so are less likely to accept a proton
How are quarternary ammonium salts used for targeted drug action?
quarternary ammonium salts have a permanent positive charge, and so cannot pass through membranes
ex. inhaled quarternary ammonium salts will stay in the lungs
Can amines be dealkylated?
secondary and tertiary (NOT primary) amines can be dealkylated (-CH3 groups removed)
**very important**
Can amines be oxidized?
secondary and tertiary (NOT primary) amines can be oxidized to form an N-oxide
**very important**
primary amines cannot be oxidized
primary amines can be ____ with pyridoxal phosphate
deaminated
methylation with methyltransferase ____ water solubility
decreases
-CH3 groups are hydrophobic
oxidation ____ water solubility
increases
conjugation to form a glucoronide ____ water solubility
increases
conjugation to form a sulfonamide ____ water solubility
increases
acetlyation ____ water solubility
decreases
Drugs that contain primary amines can have their water solubility increased by:
A. Methylation to form a secondary amine
B. Acetylation to form an amide
C. Oxidation to form an N-oxide
D. Conjugation to form a glucuronide
Drugs that contain primary amines can have their water solubility increased by:
A. Methylation to form a secondary amine decreases solubility
B. Acetylation to form an amide decreases solubility
C. Oxidation to form an N-oxide primary amines cannot be oxidized
D. CONJUGATION TO FORM A GLUCURONIDE
carboxylic acids
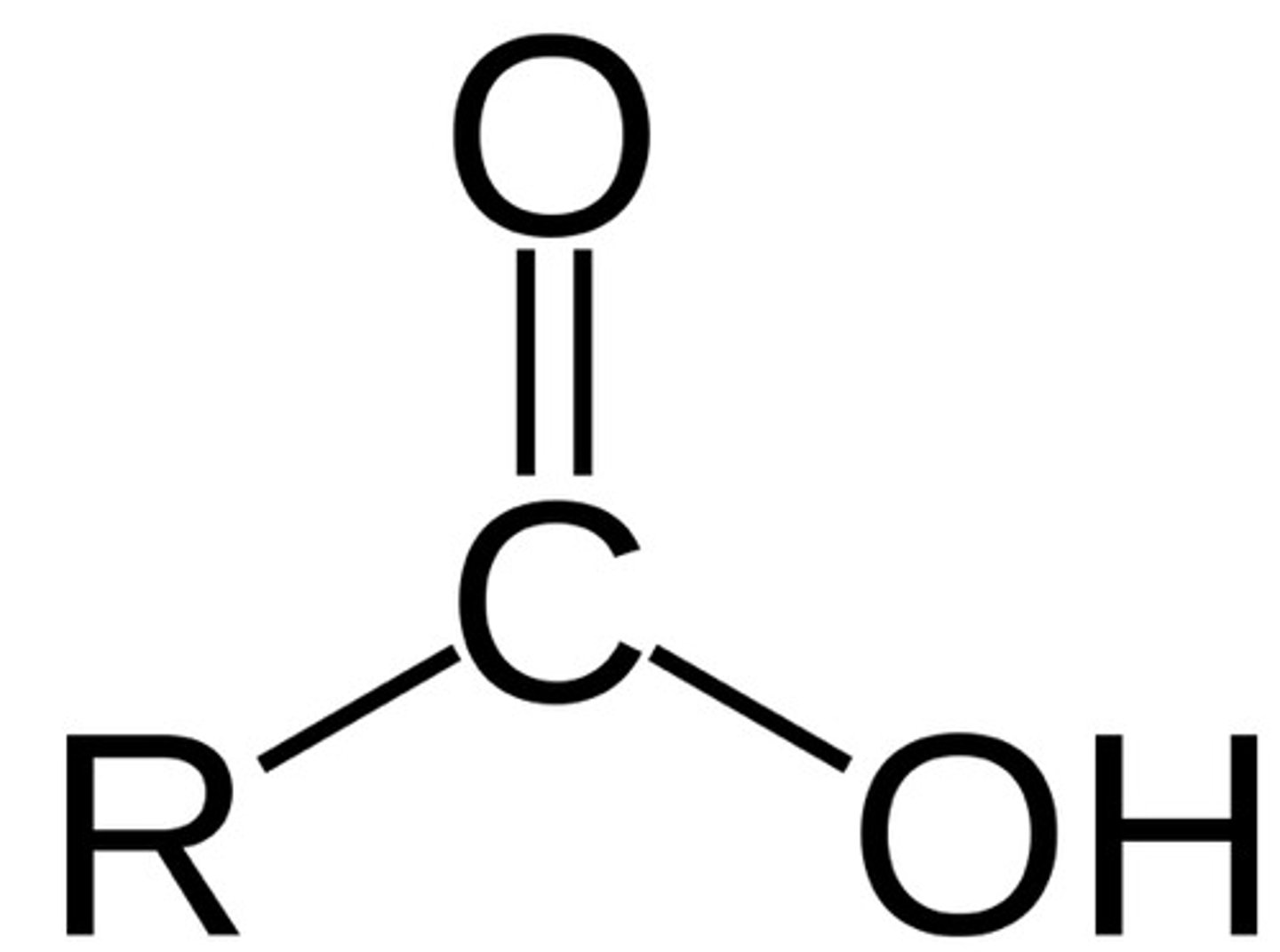
formic acid
carboxylic acid and toxic byproduct of methanol oxidation
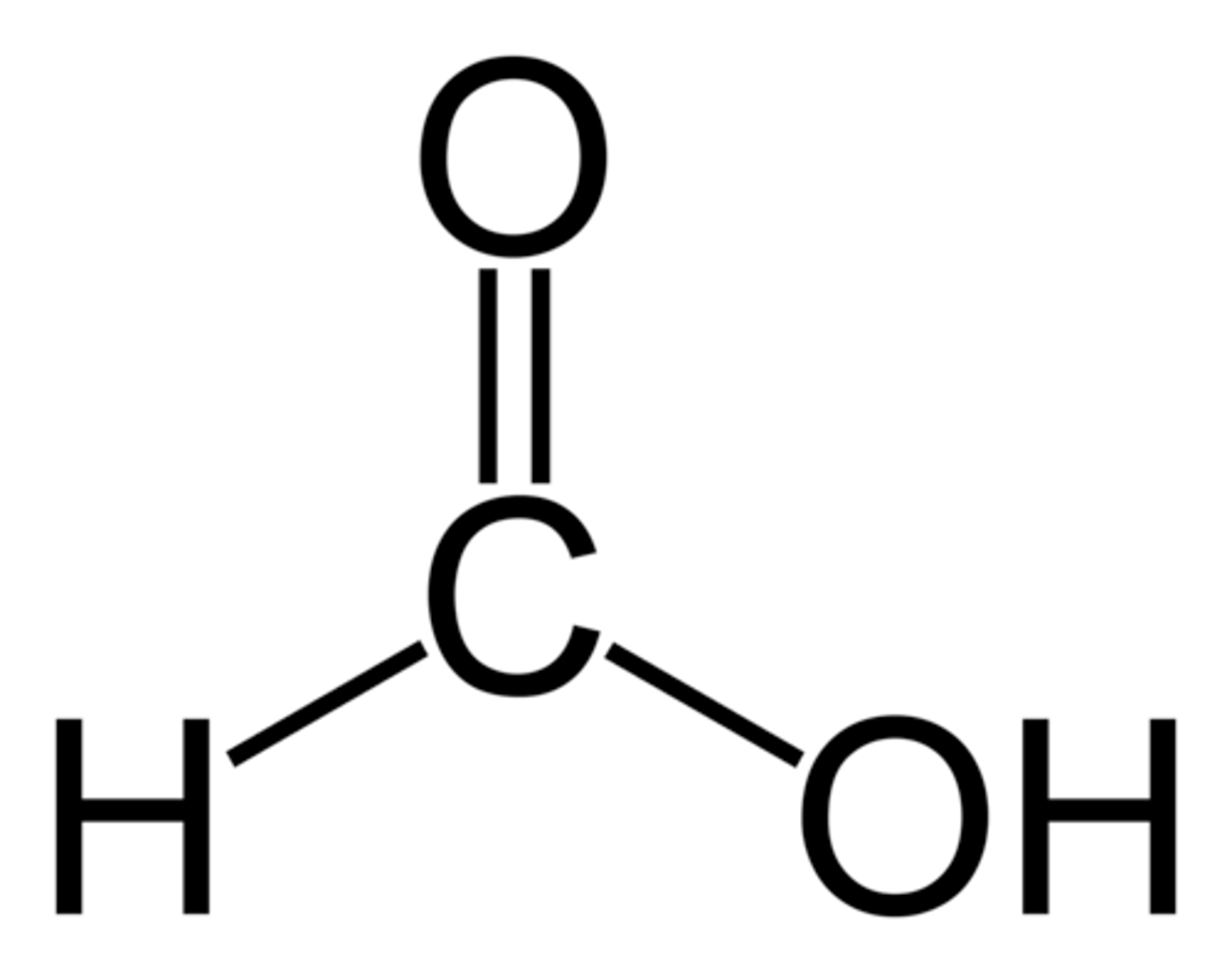
Can carboxylic acids hydrogen bond with each other?
yes
results in characteristically high boiling point and water solubility
How can carboxylic acids with long alkyl chains be solvated?
the alkyl chains can vdWaals with each other to decrease solubility, so use a mixture of ethanol and H2O
the ethanol forms vdWaals with the alkyl chain, and the water forms H-bonds with the carboxylic acid
naturally-occurring unsaturated fatty acids exist in ____ conformation
cis
beta oxidation of carboxylic acids is the systematic removal of ____
two carbons at a time to form a C(n-2) chain and acetyl-CoA
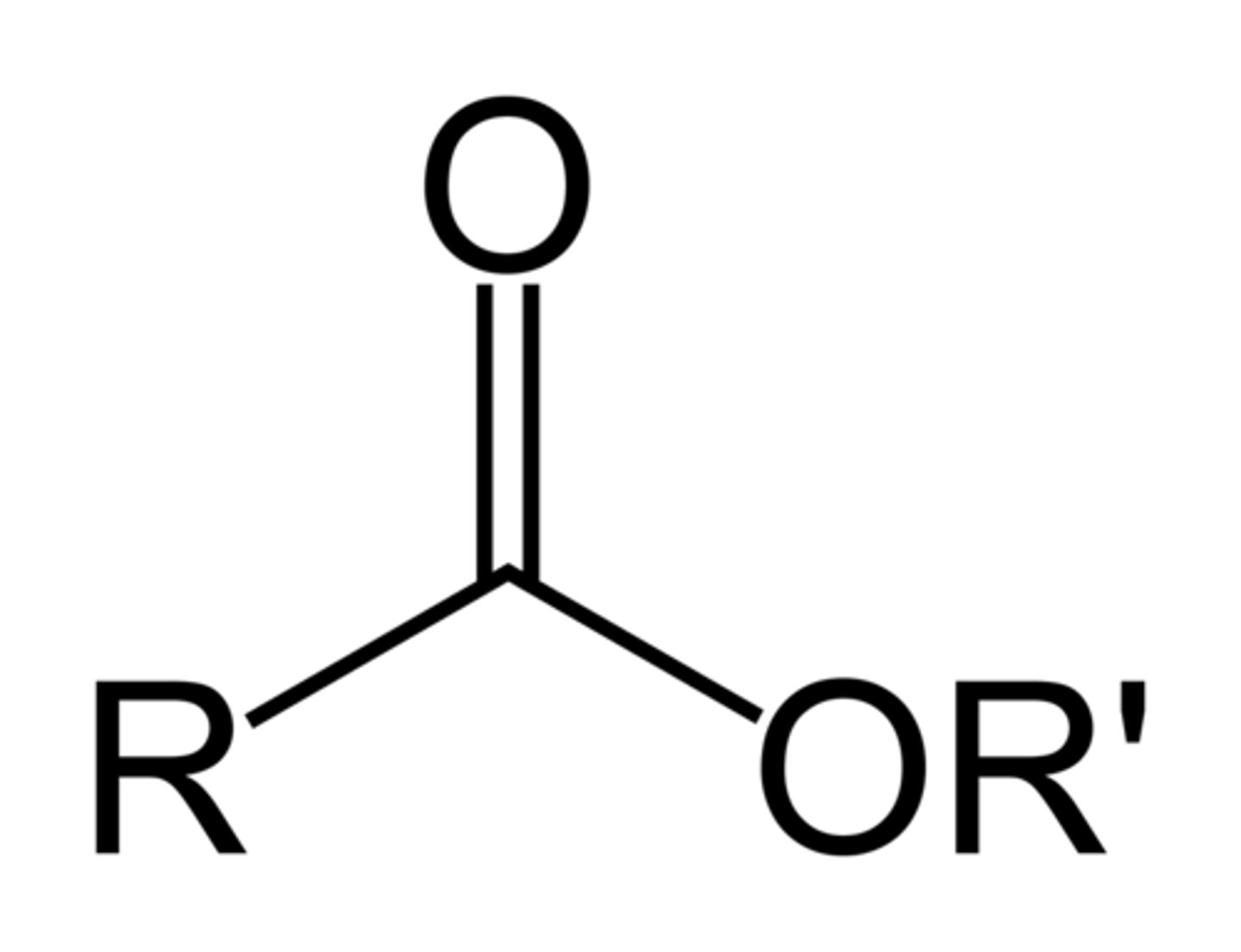
esters
carboxylic acid + O-alkyl
lactones
cyclic esters
Can esters hydrogen bond with each other?
no
results in low boiling point due to weak intermolecular forces and low-ish half-life
esters are susceptible to ____ in vitro
hydrolysis to form carboxylic acid + alcohol
half-life of novocaine metabolite
1.5 minutes (contains an ester, so low-ish half-life)
(for some reason she said to remember this....)
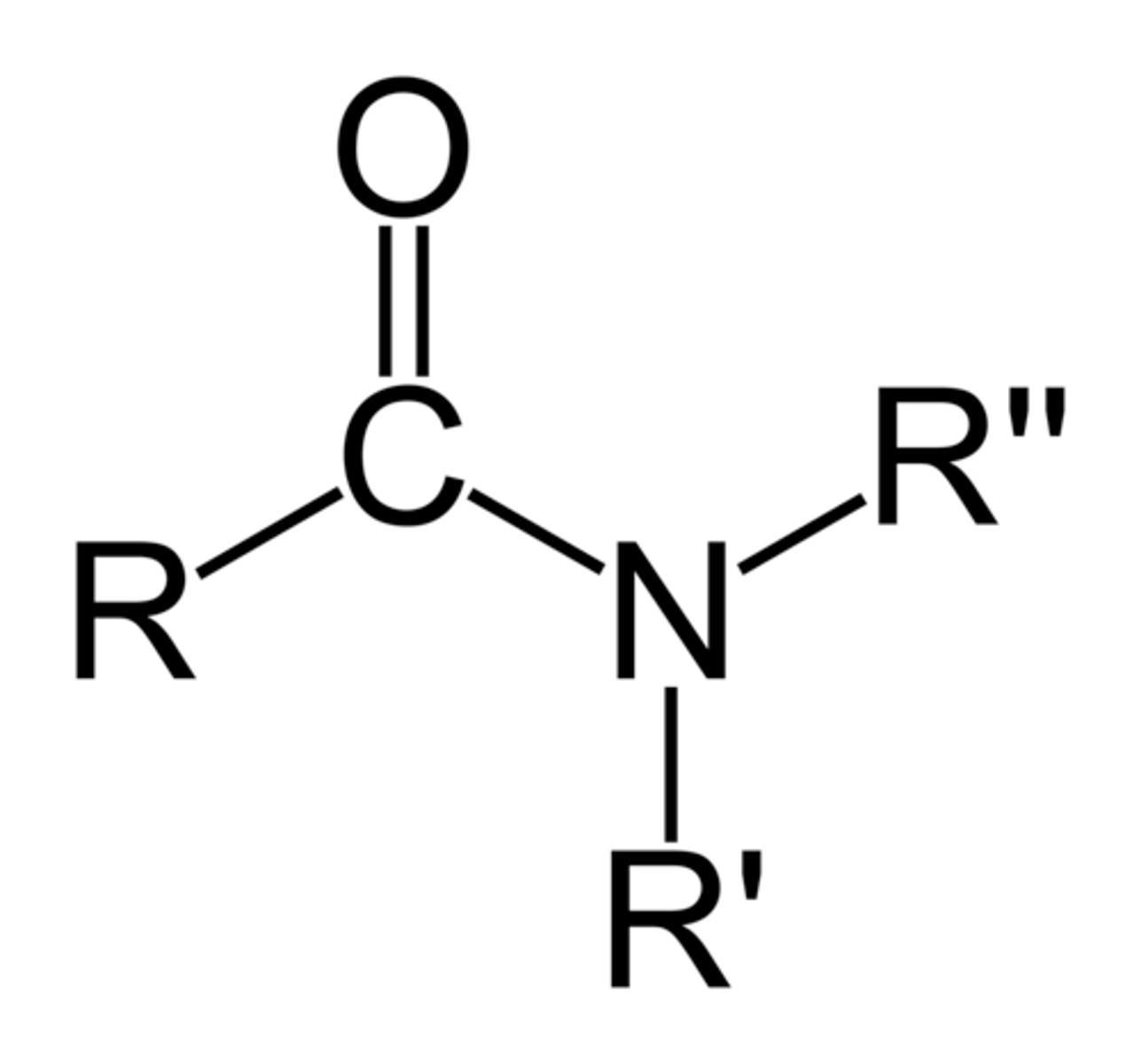
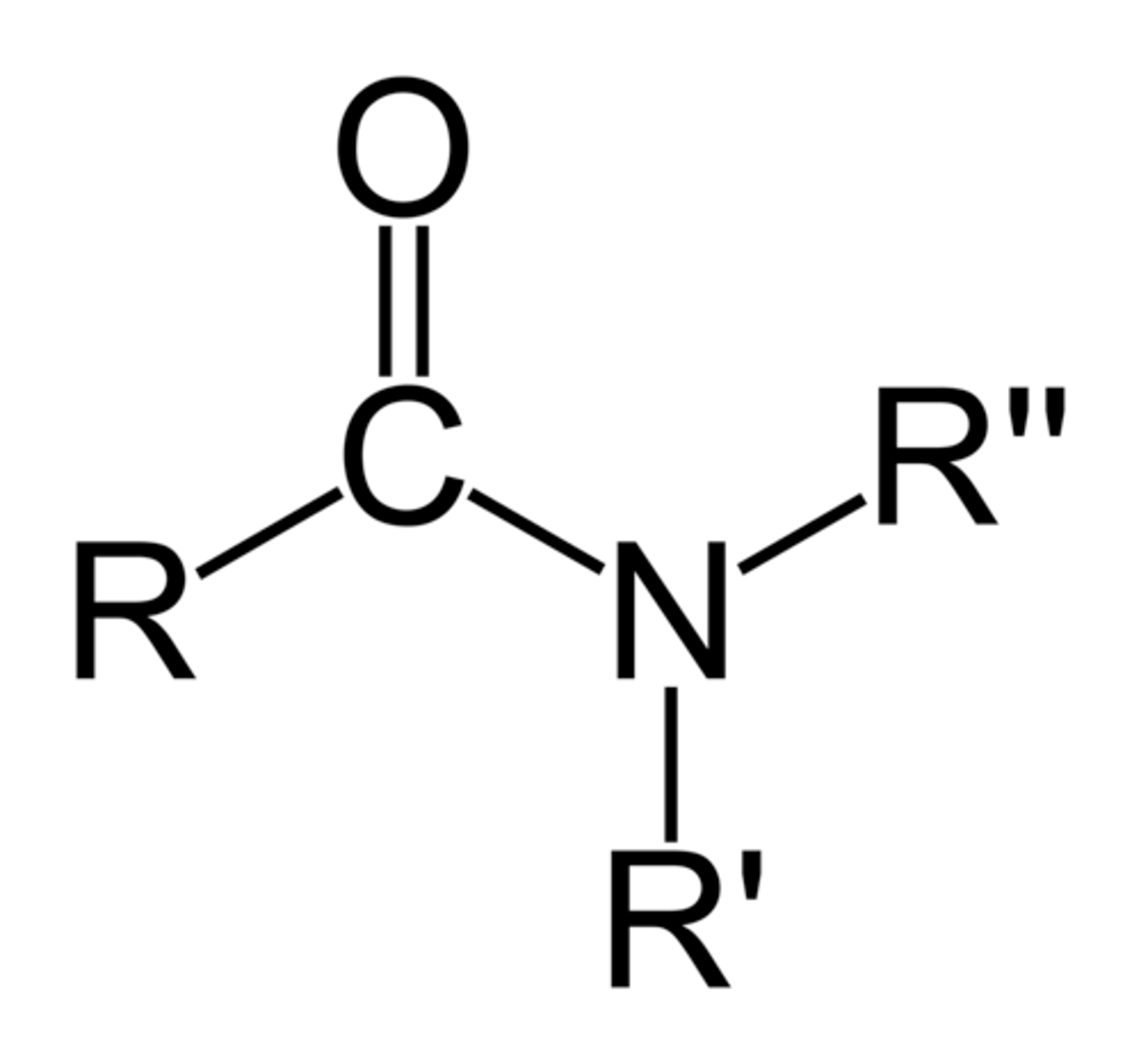
amides
carboxylic acid + amine
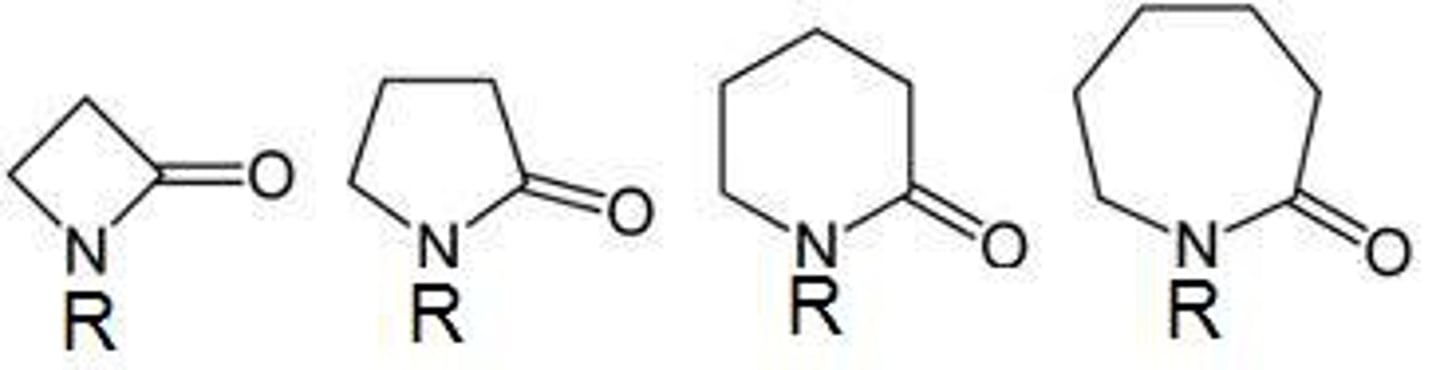
lactams
cyclic amides
very reactive
Can amides hydrogen bond?
if R' or R" is a hydrogen, then yes
boiling point of amides vs esters
amides have a higher BP due to stronger intermolecular forces --> longer half-life
amides can resonate --> ion-dipole interactions (which are stronger than H-bonds)
Which has longer half-life: esters or amides?
amides
amides can hydrogen bond with each other if one of the R'/R" are a hydrogen
--> changing an ester for an amide will result in longer activity
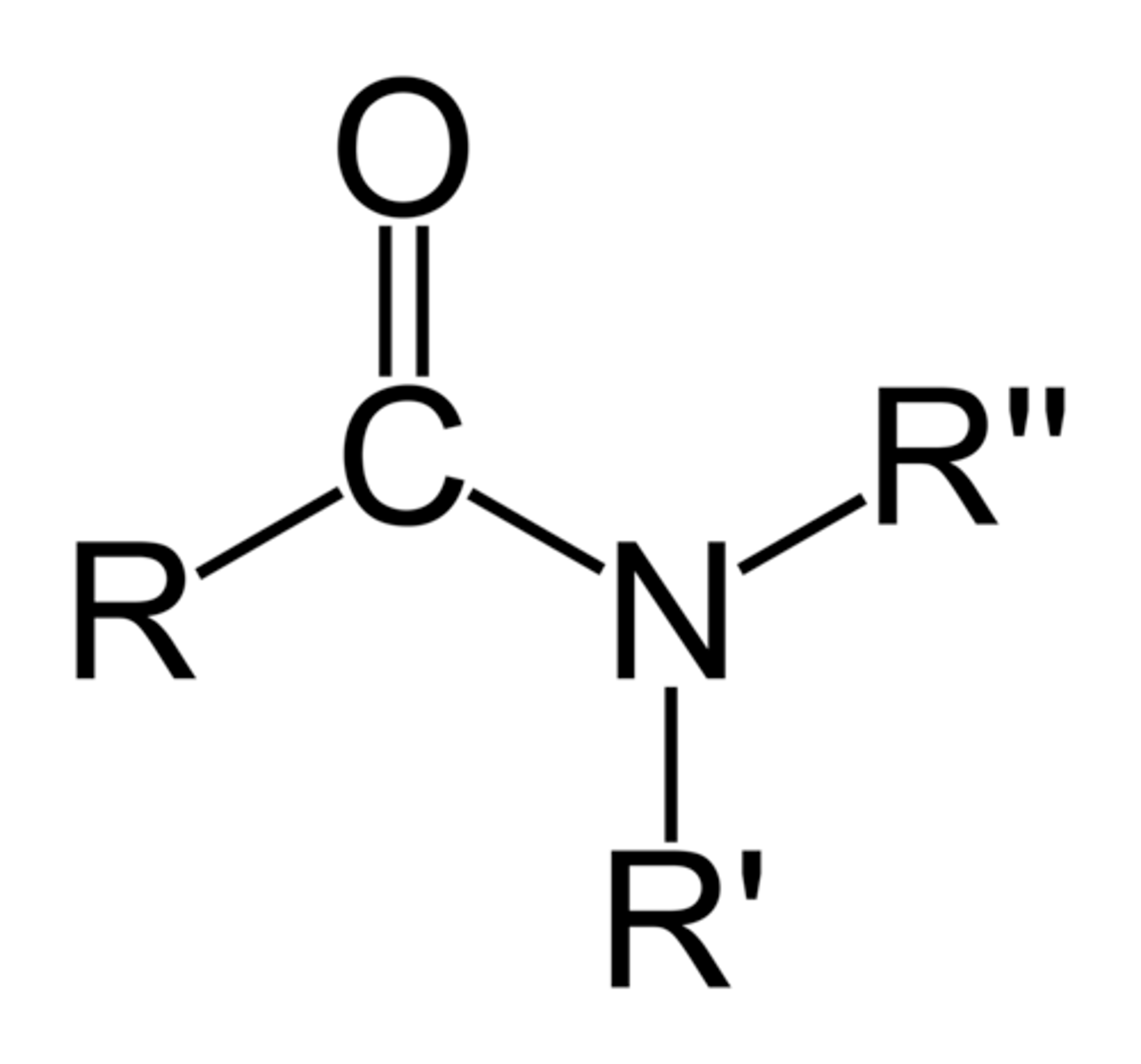
Are amides acidic or basic?
neutral (not acidic or basic)
What is the product of amide hydrolysis?
carboxylic acid + amine
carbonate
contains a diester (C=O sandwiched between two O-R groups)
carbamate
hydrolysis --> 1 alcohol + 1 amine + CO2
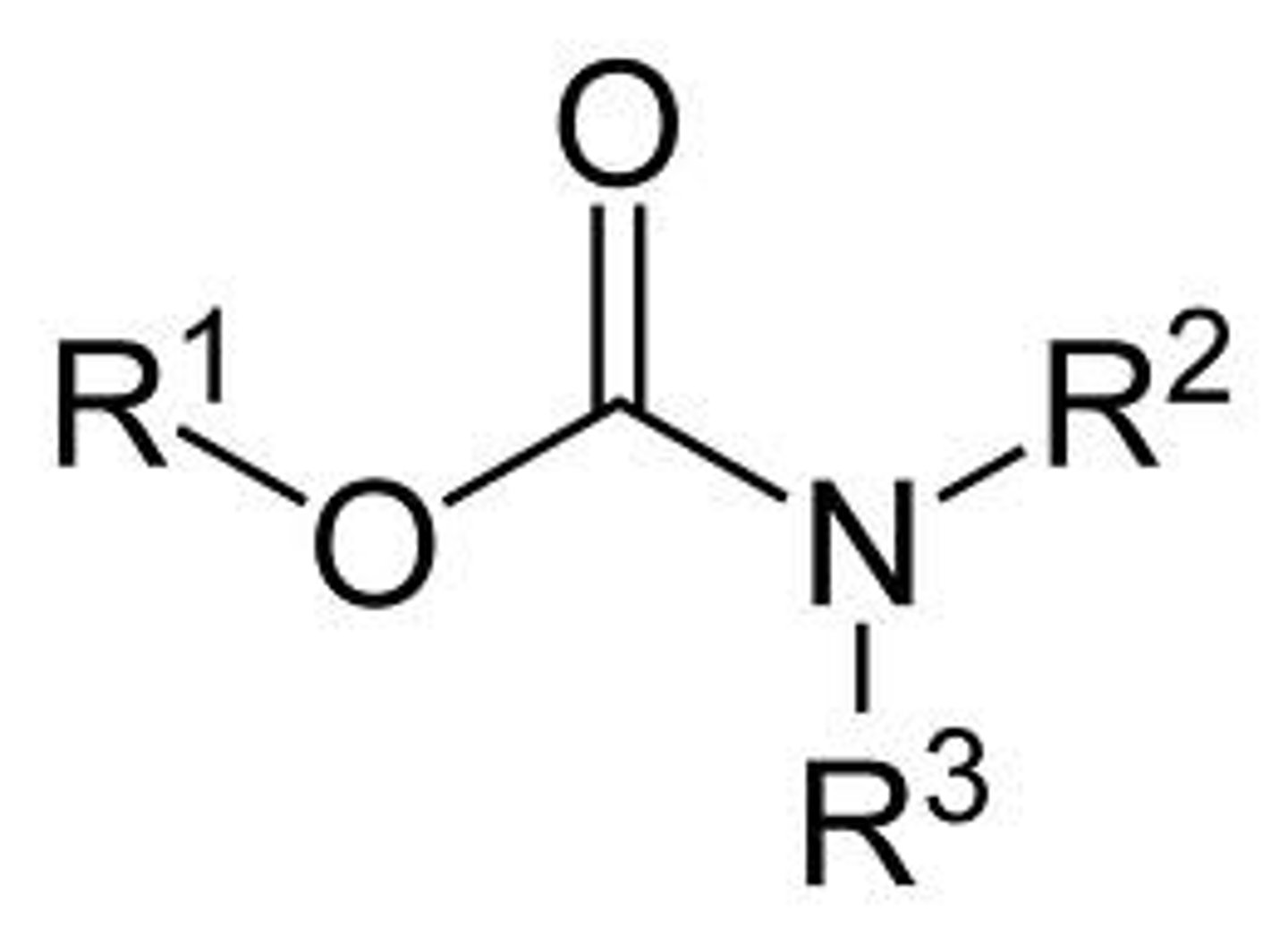
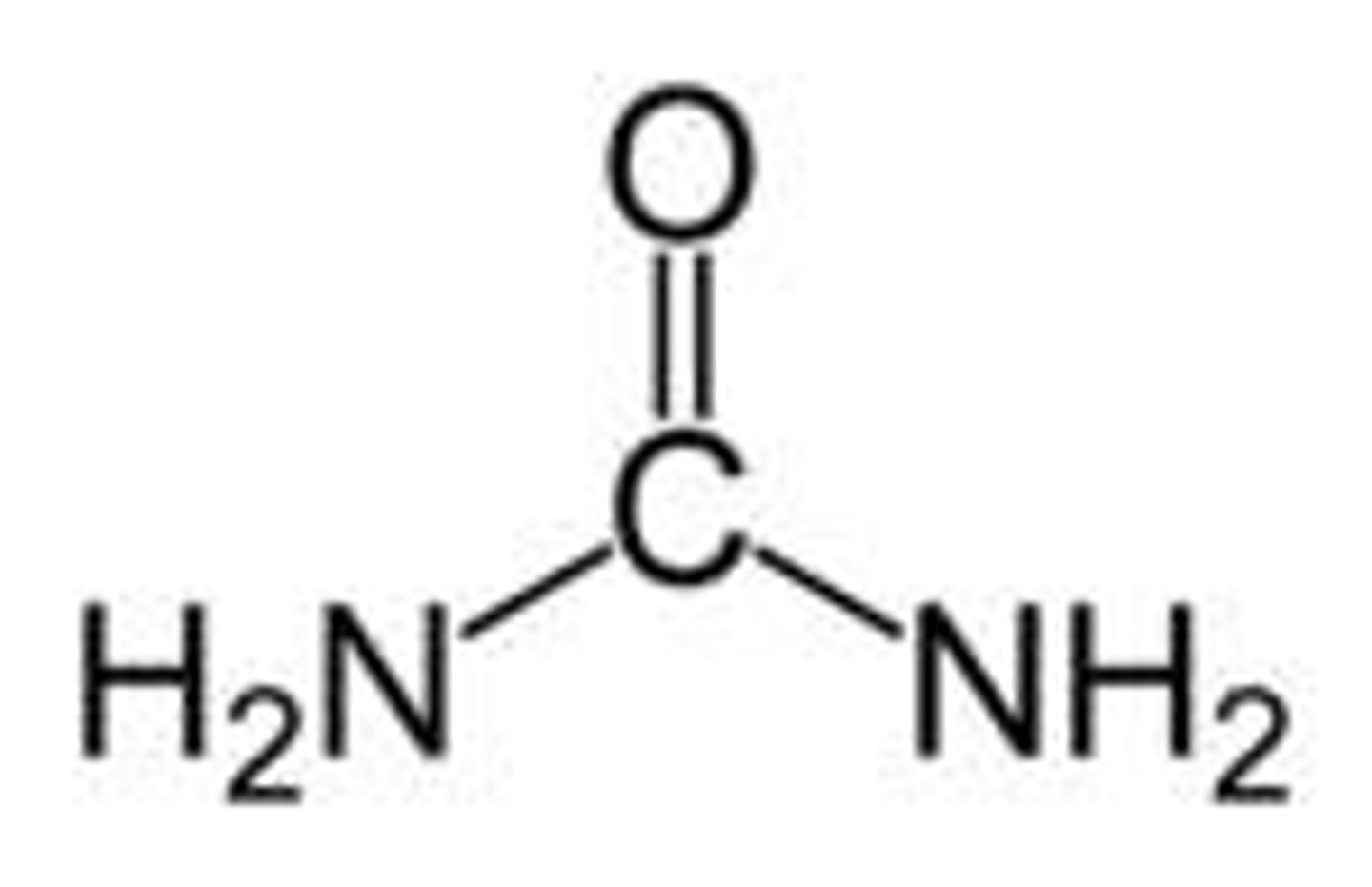
urea
diamide
relatively nonreactive to esterases
metabolic products of carbonate + esterase
2 alcohols + CO2
metabolic products of carbamate + esterase
1 alcohol + 1 amine + CO2
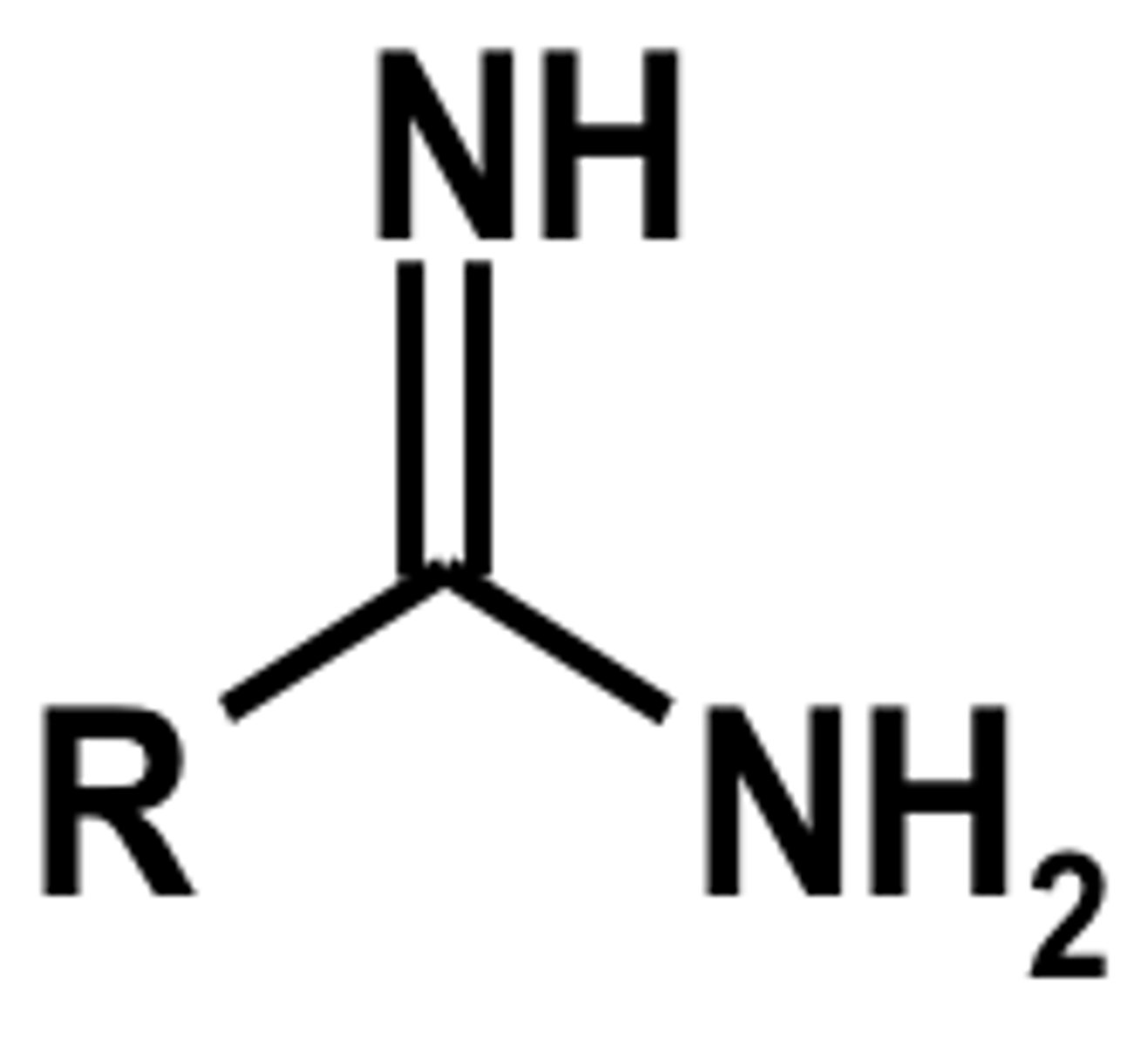
amidine
contains an imine (C=NH) bond
guanidine
derivative of urea
very basic
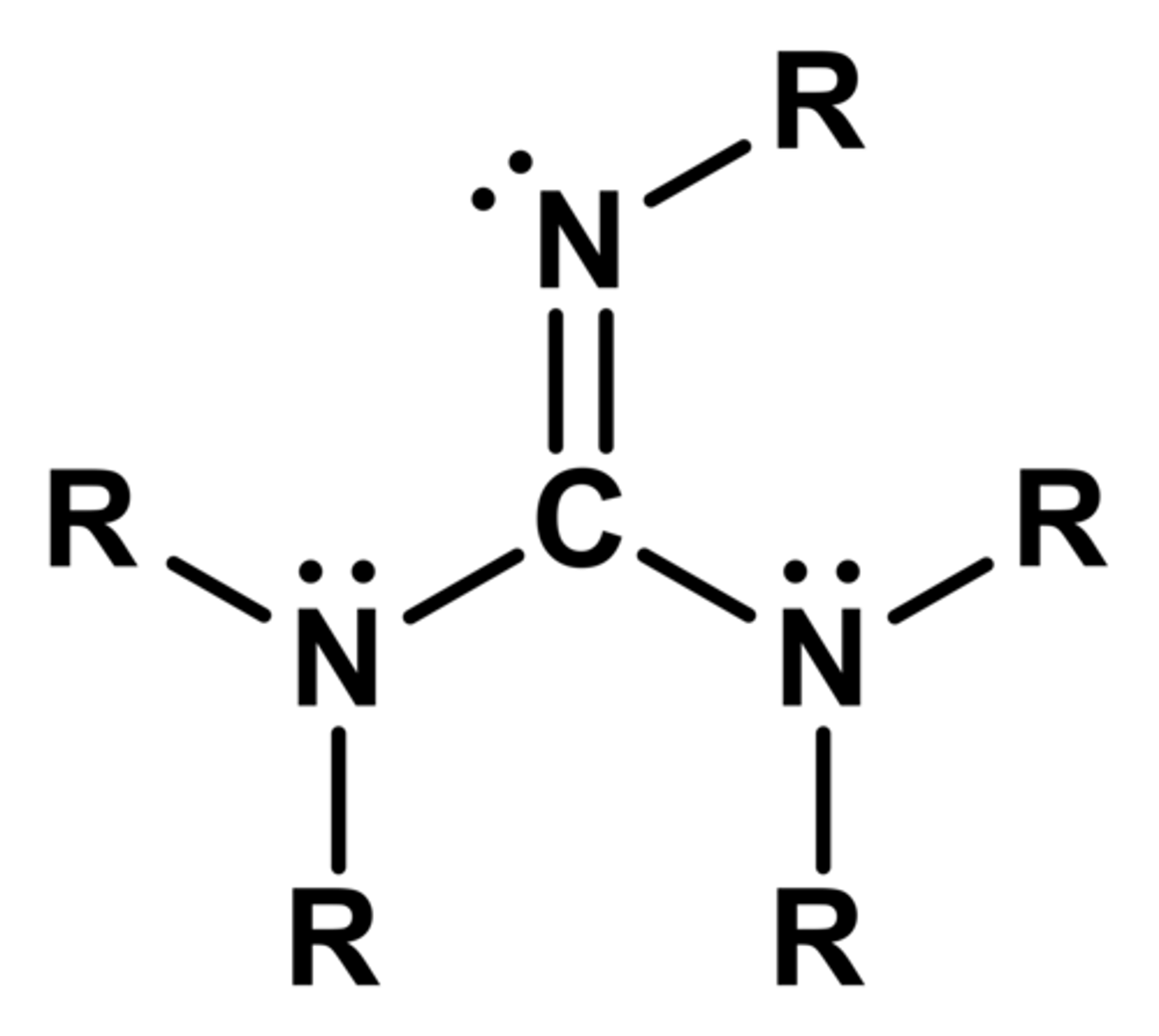
Are amidines and guanidines acidic or basic?
basic
conjugate base is stabilized by resonance due to imine nitrogen (C=NH)
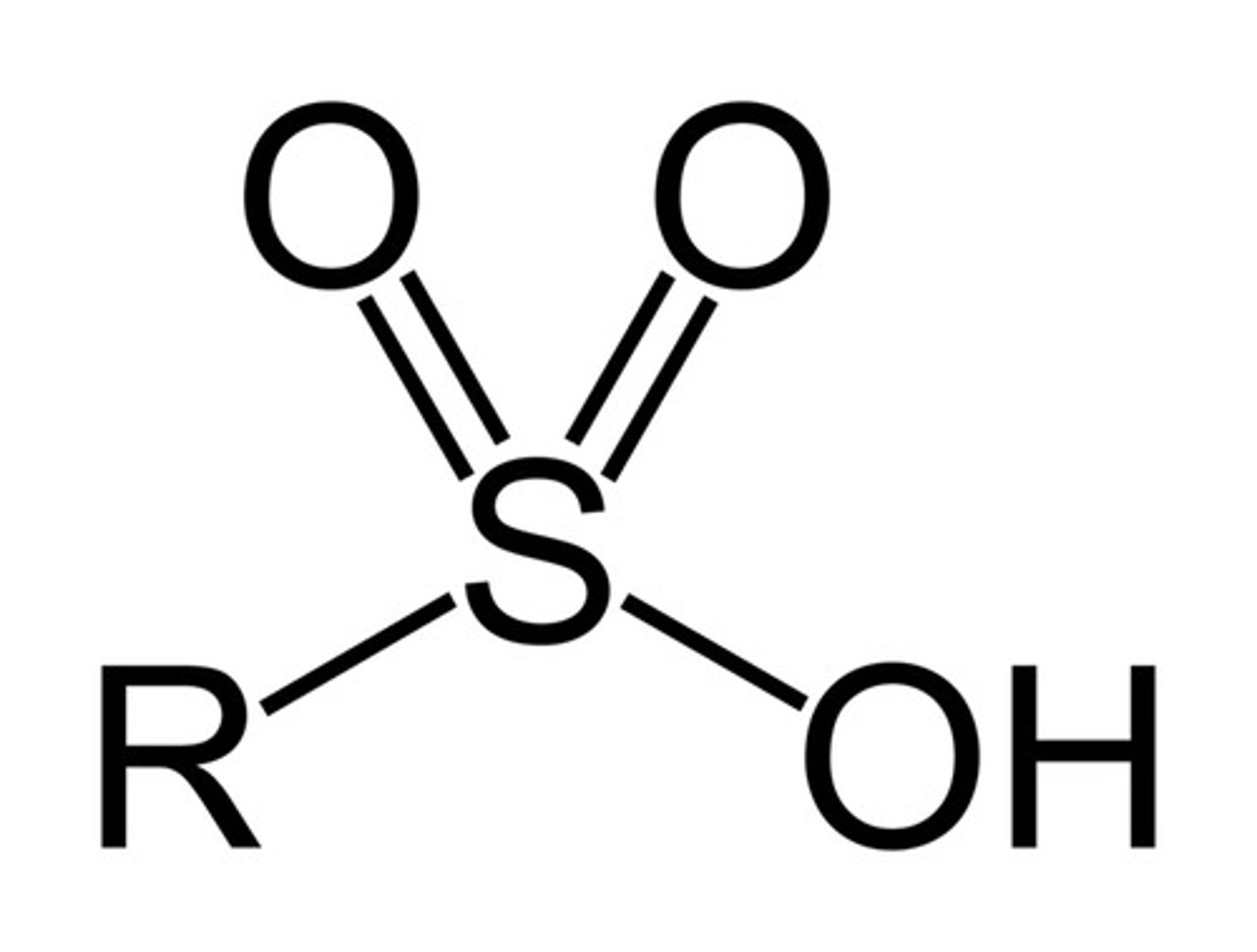
sulfonic acid
-SO3H
very acidic
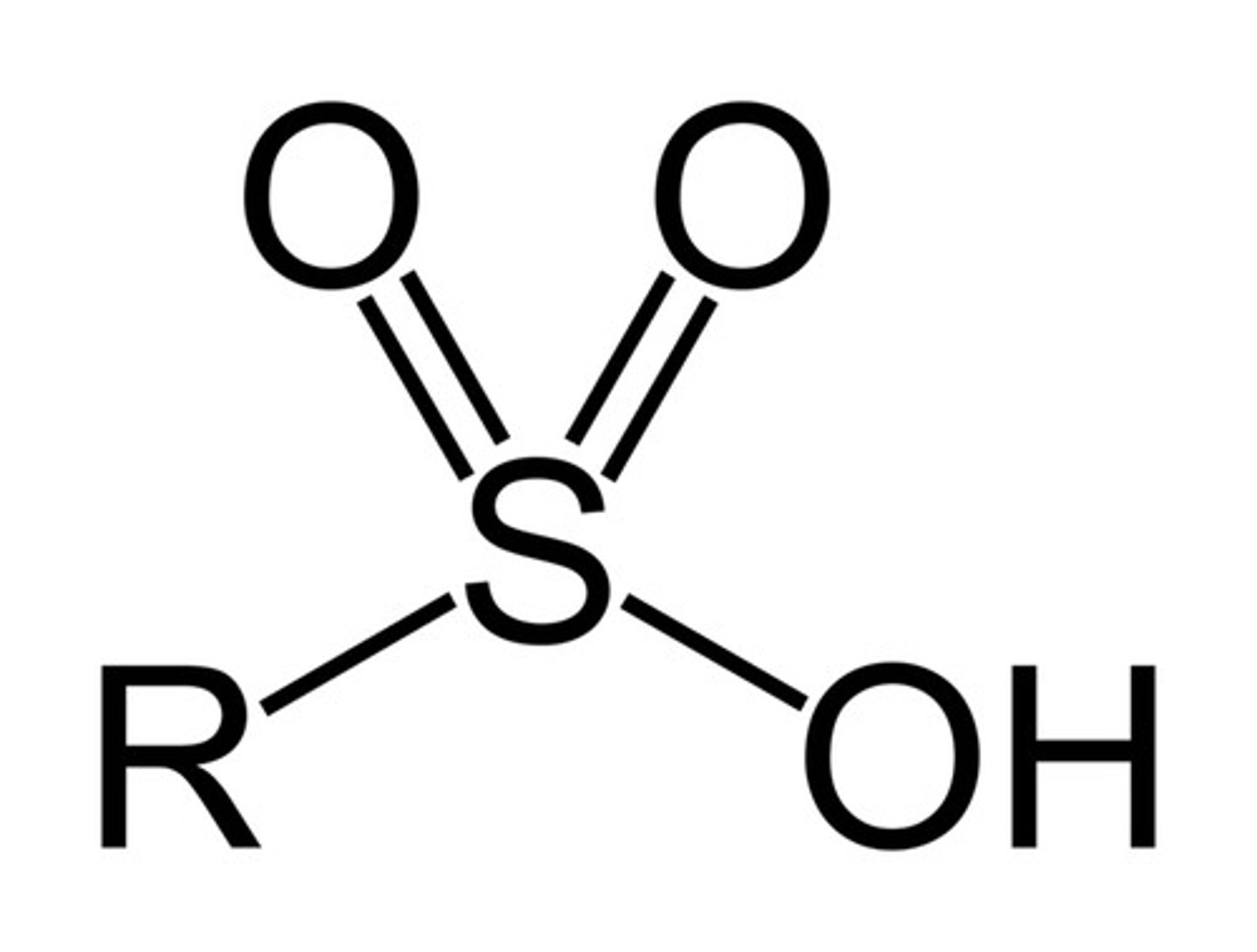
Are sulfonic acids acidic or basic?
acidic (obviously....)
-SO2 groups are electron-withdrawing, so electron density is dispersed
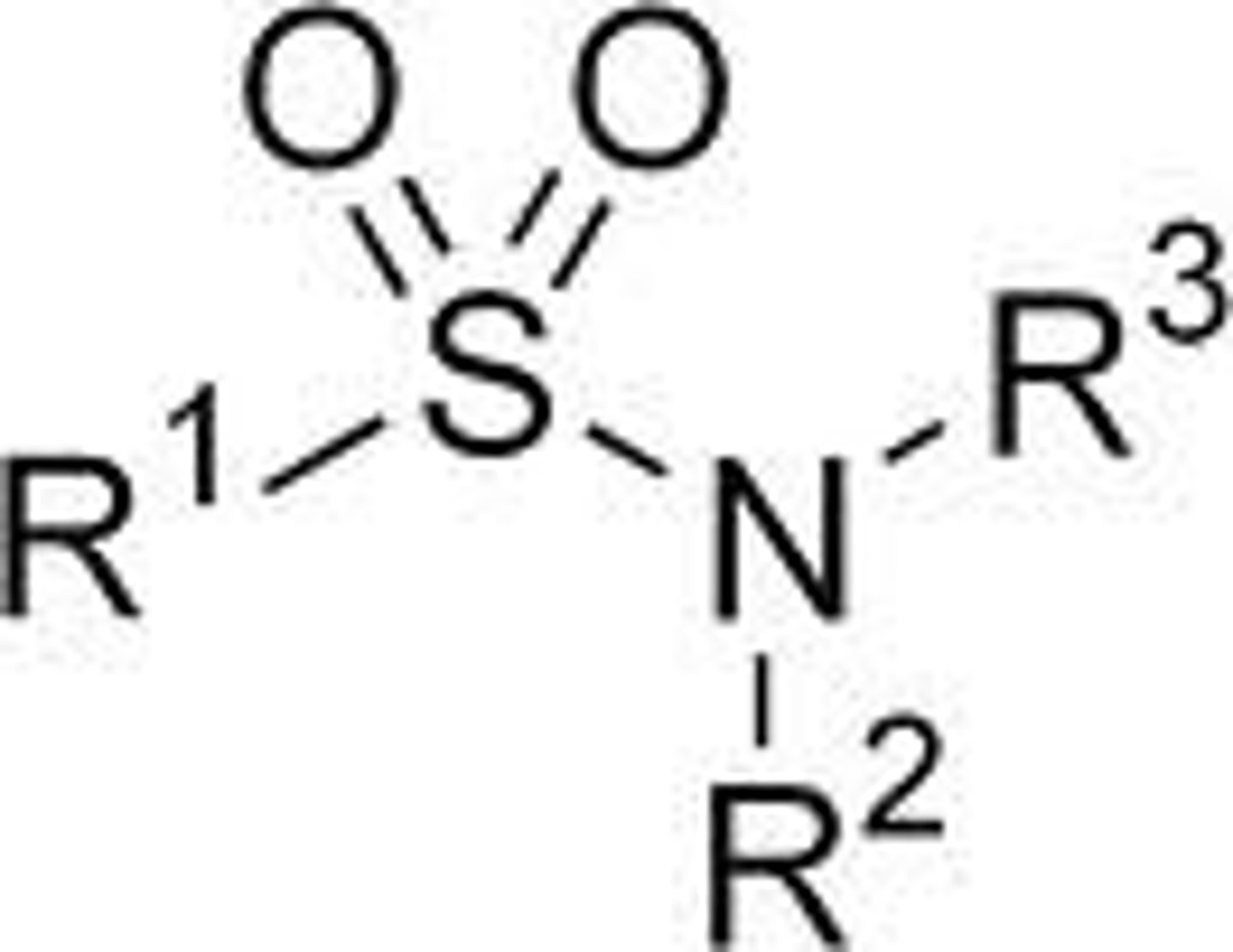
sulfonamides
contains SO2 + amine
weakly acidic
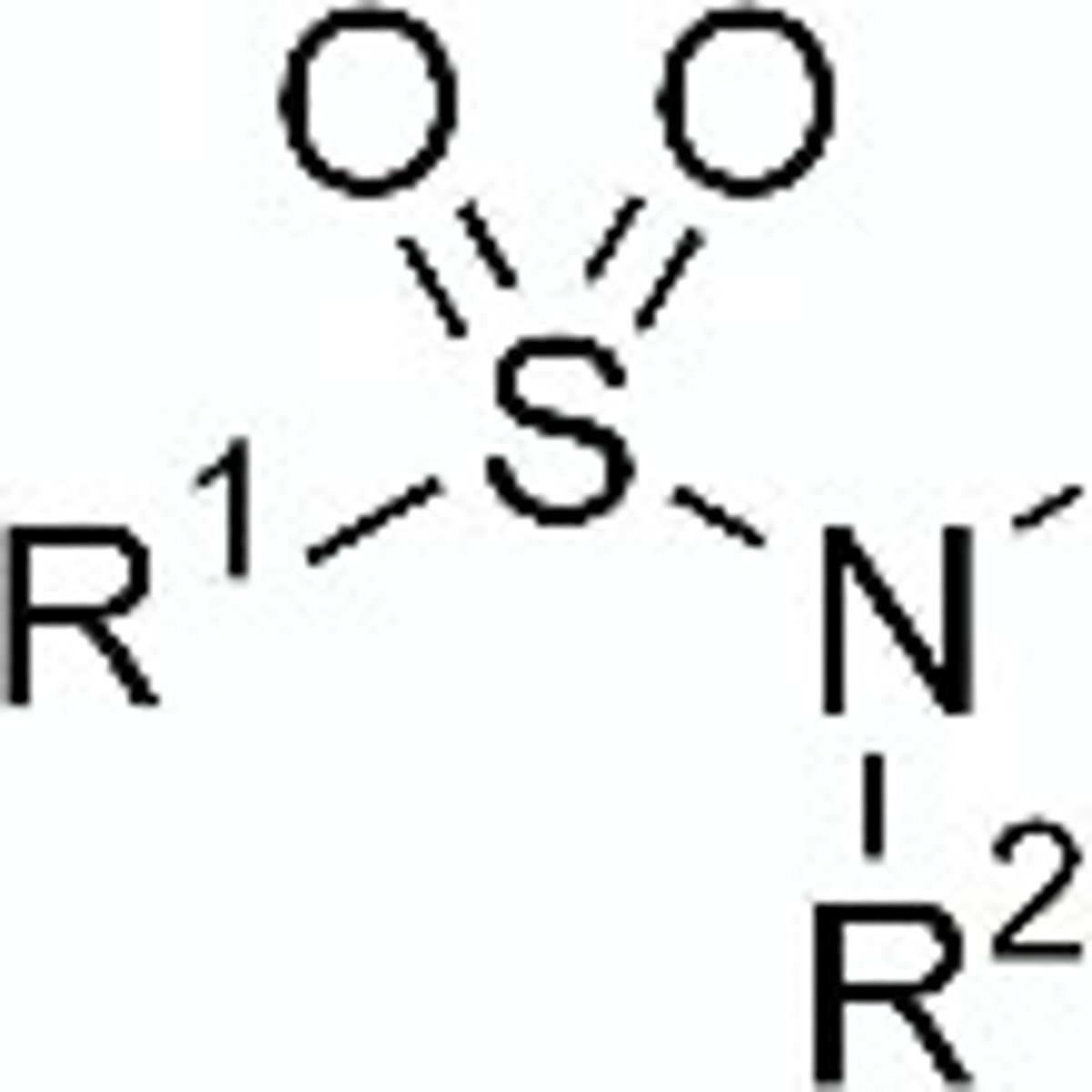
Are sulfonamides acidic or basic?
weakly acidic due to resonance stability of conjugate acid
-SO2 groups are very electron-withdrawing, which counteracts the basic property of the amine group
nitro groups
-NO2
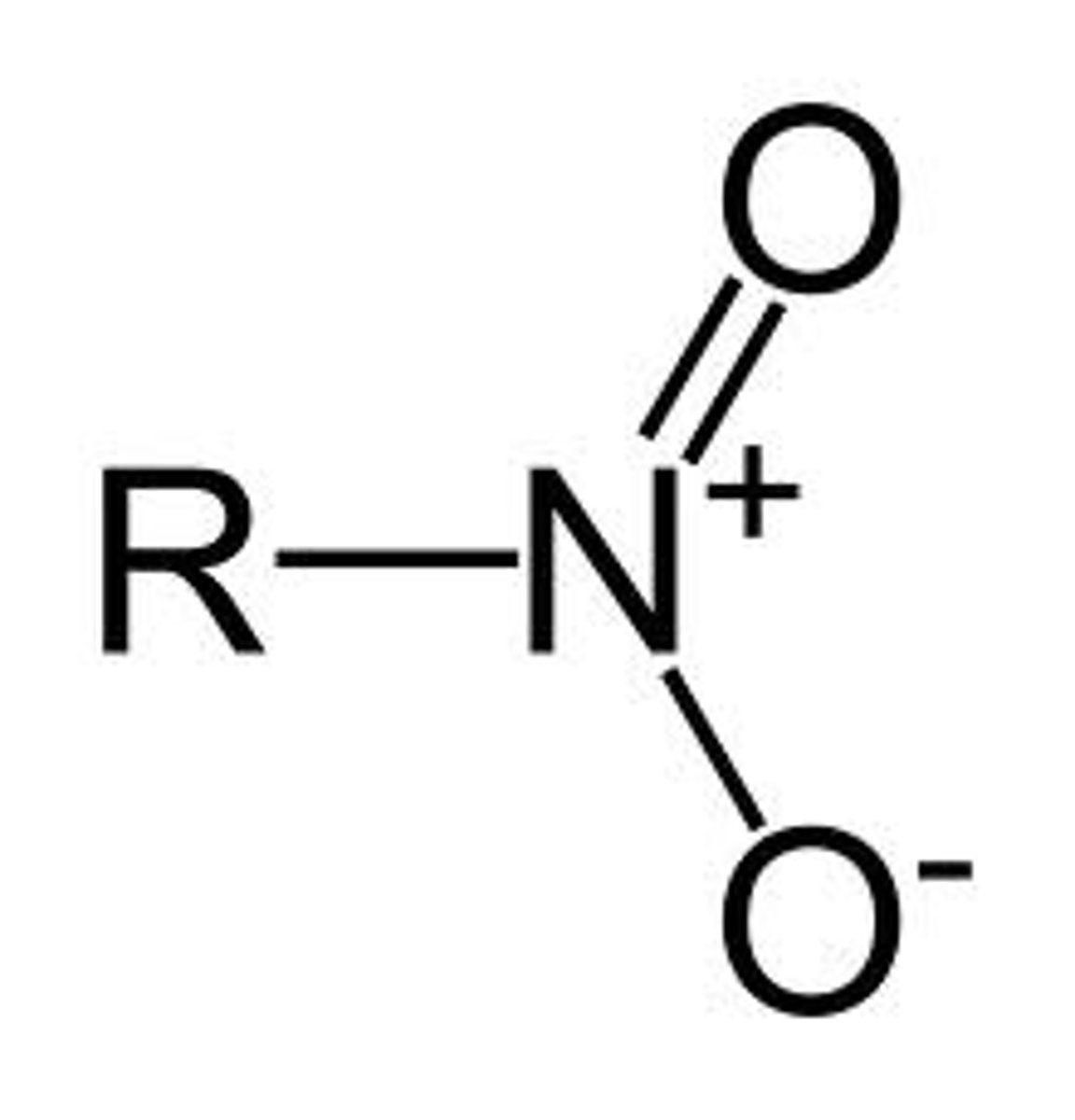
Are nitro groups acidic or basic?
neutral, but electron-withdrawing
EW nature causes other groups to be acidic
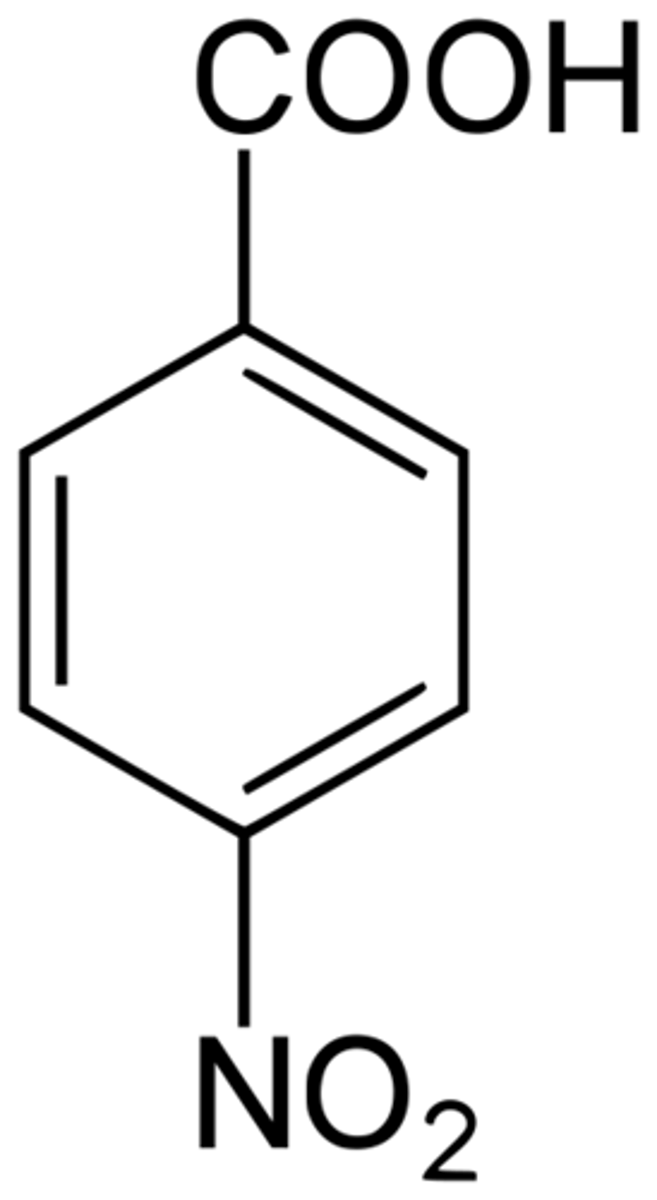
Compare the acidity of para-nitro vs meta-nitro substituents on benzoic acid
para position is more acidic due to resonance stability of conjugate acid
the negative charge on the CA is better dispersed throughout the ring for para-nitro
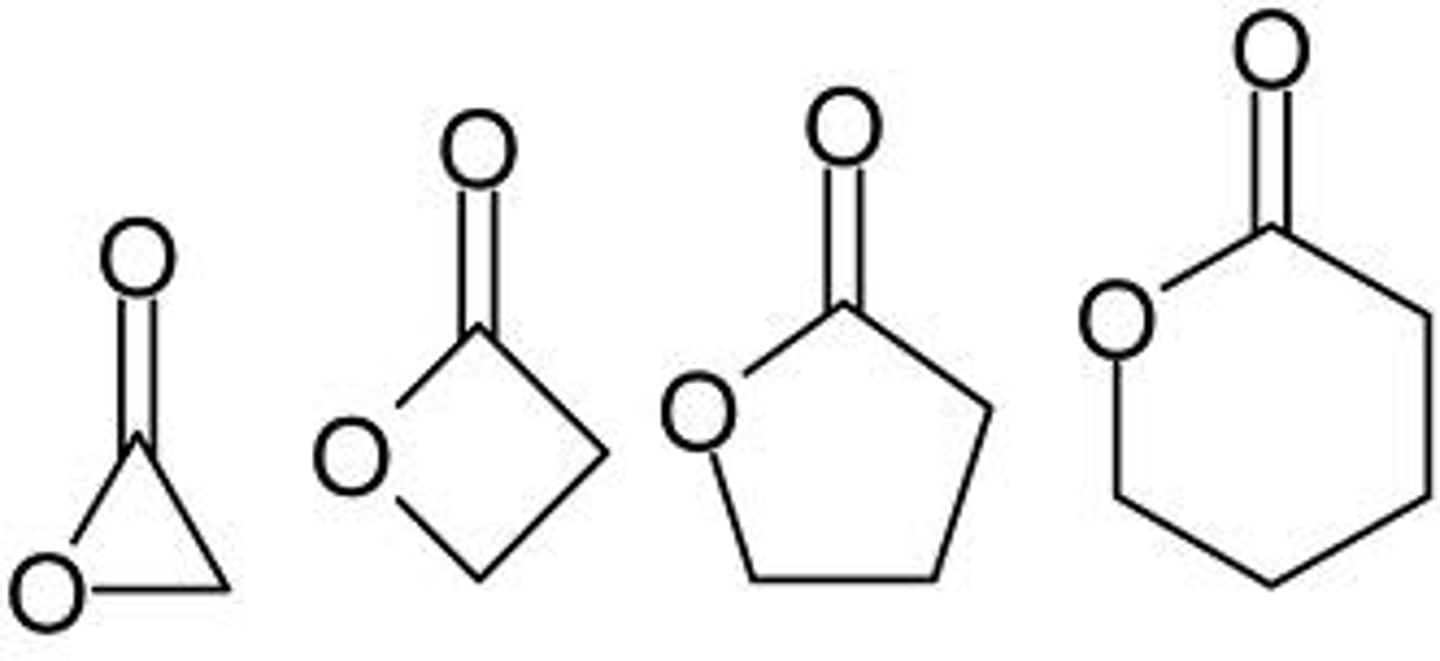
heterocycle
ring structure that contains at least two different elements in the ring (oxygen, nitrogen, or sulfur)
oxa- heterocycle
contains oxygen
aza- heterocycle
contains nitrogen
thia- heterocycle
contains sulfur
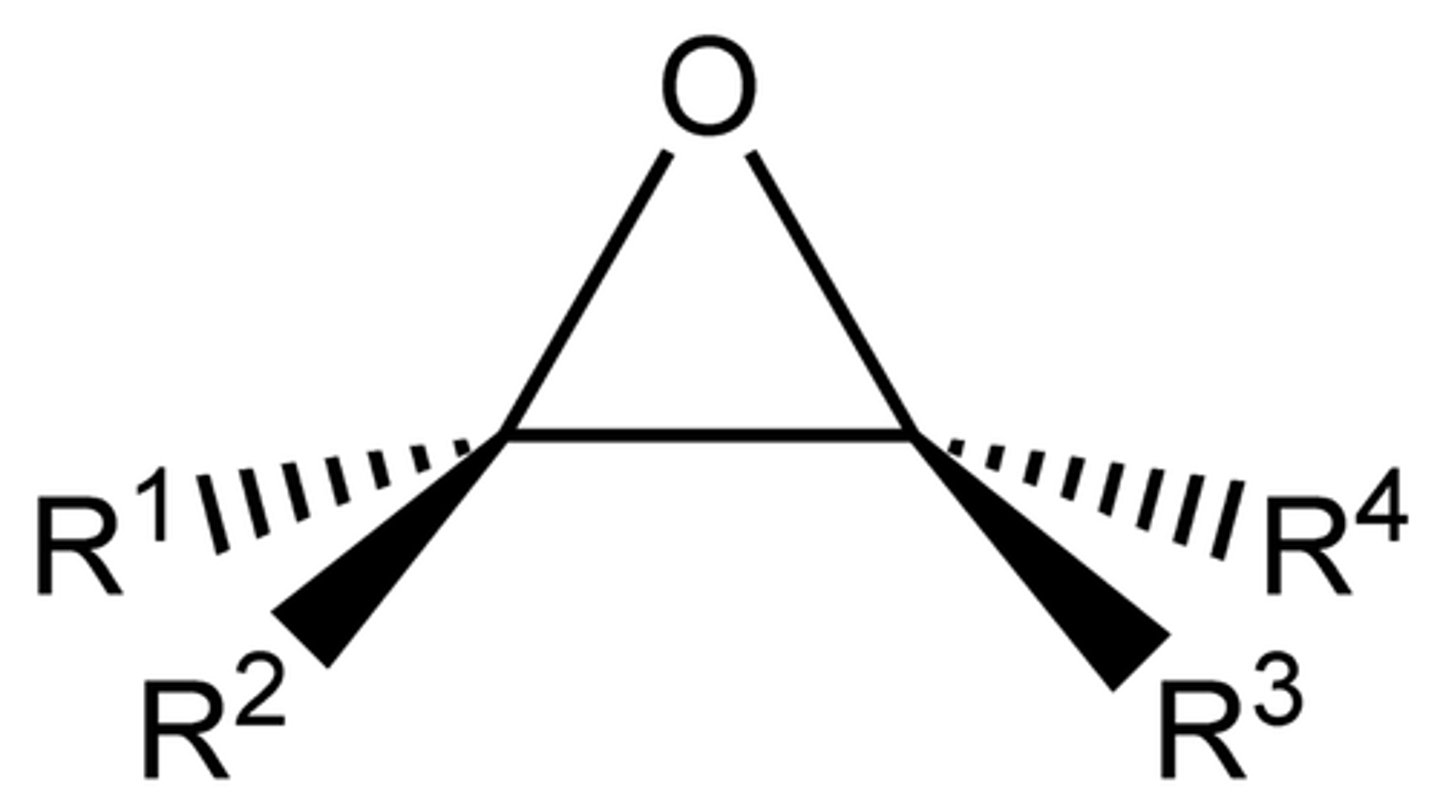
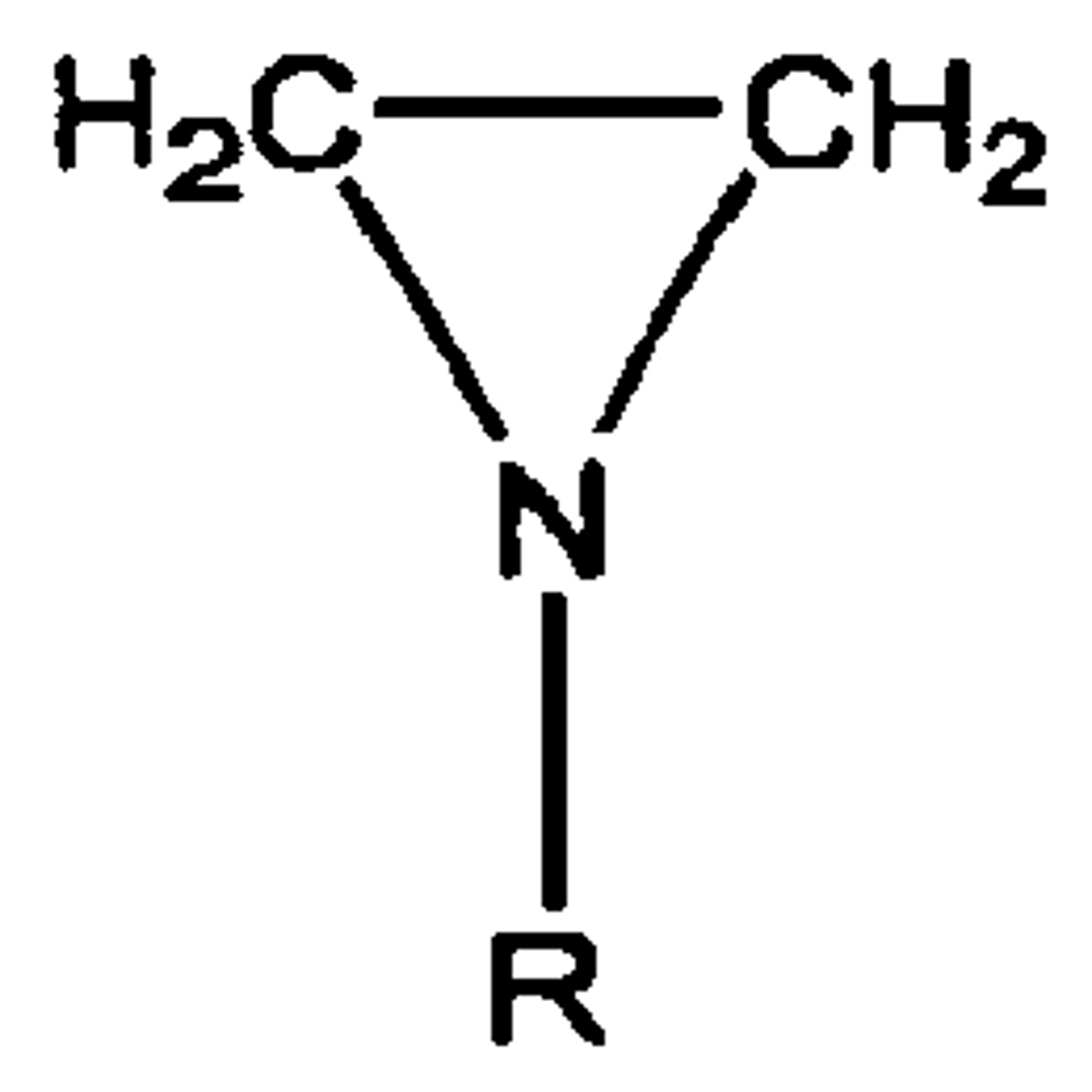
Why are epoxides and azirides unstable?
60-degree angle of C-O/N bonds causes significant ring strain, making them very reactive to nucleophiles
Ethylene oxide is more reactive than dimethyl ether because of:
A. The availability of the electrons on the oxygen atom
B. Ring strain
C. The difference in electron-donating ability of CH2 vs CH3
D. The difference in electron-withdrawing ability of CH2 vs CH3
Ethylene oxide is more reactive than dimethyl ether because of:
A. The availability of the electrons on the oxygen atom
B. RING SRAIN
C. The difference in electron-donating ability of CH2 vs CH3
D. The difference in electron-withdrawing ability of CH2 vs CH3
aziride ring
like an epoxide, but the O is replaced with N
beta-lactam
4-membered ring with nitrogen and C=O
ring straing --> very reactive
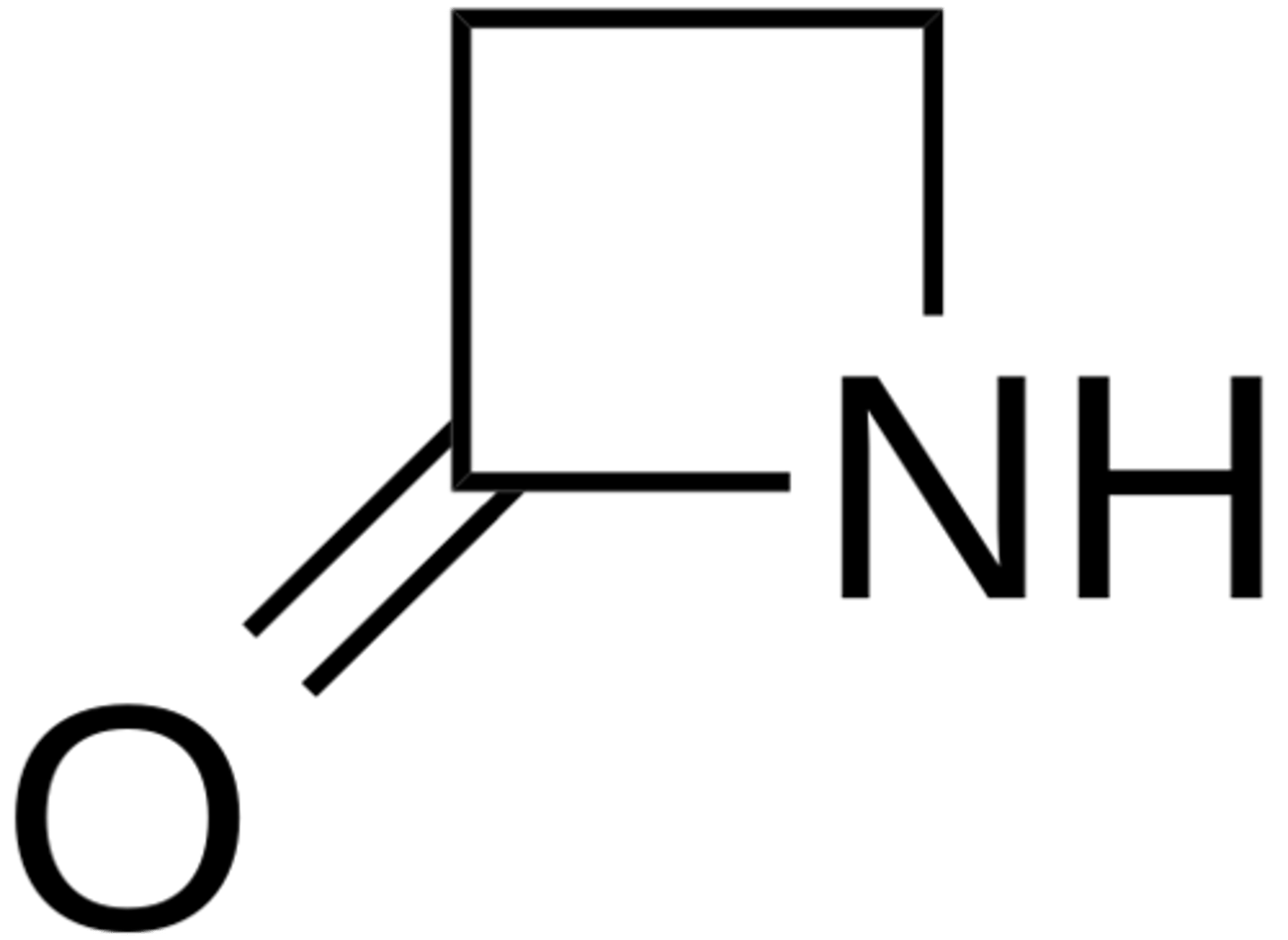
How can bacteria become resistant to B-lactam antibiotics?
some bacteria produce B-lactamase, which hydrolyzes B-lactams to inactivate the antibiotic