Enzyme Inhibition Types: Reversible, Irreversible, and Kinetics, Week 5 - Cell Signaling Receptors: Types and Mechanisms ,Cell Signaling Pathways
1/78
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
79 Terms
What factors can affect enzyme activity?
Temperature, pH, substrate concentration, and the presence of inhibitors or activators.
What is enzyme specificity?
The ability of an enzyme to select and catalyze a specific substrate among many.
What are substances that reduce the catalytic activity of enzymes called?
Inhibitors
What type of inhibition allows enzyme activity to recover when the inhibitor is diluted out?
Reversible Inhibition
What type of inhibition results in permanent loss of enzyme activity even after dilution?
Irreversible Inhibition
What is the most common type of enzyme inhibitor that competes with the substrate for the active site?
Competitive Inhibition
How does the presence of a competitive inhibitor affect the Km and Vmax of an enzyme?
Km is increased; Vmax remains unchanged.
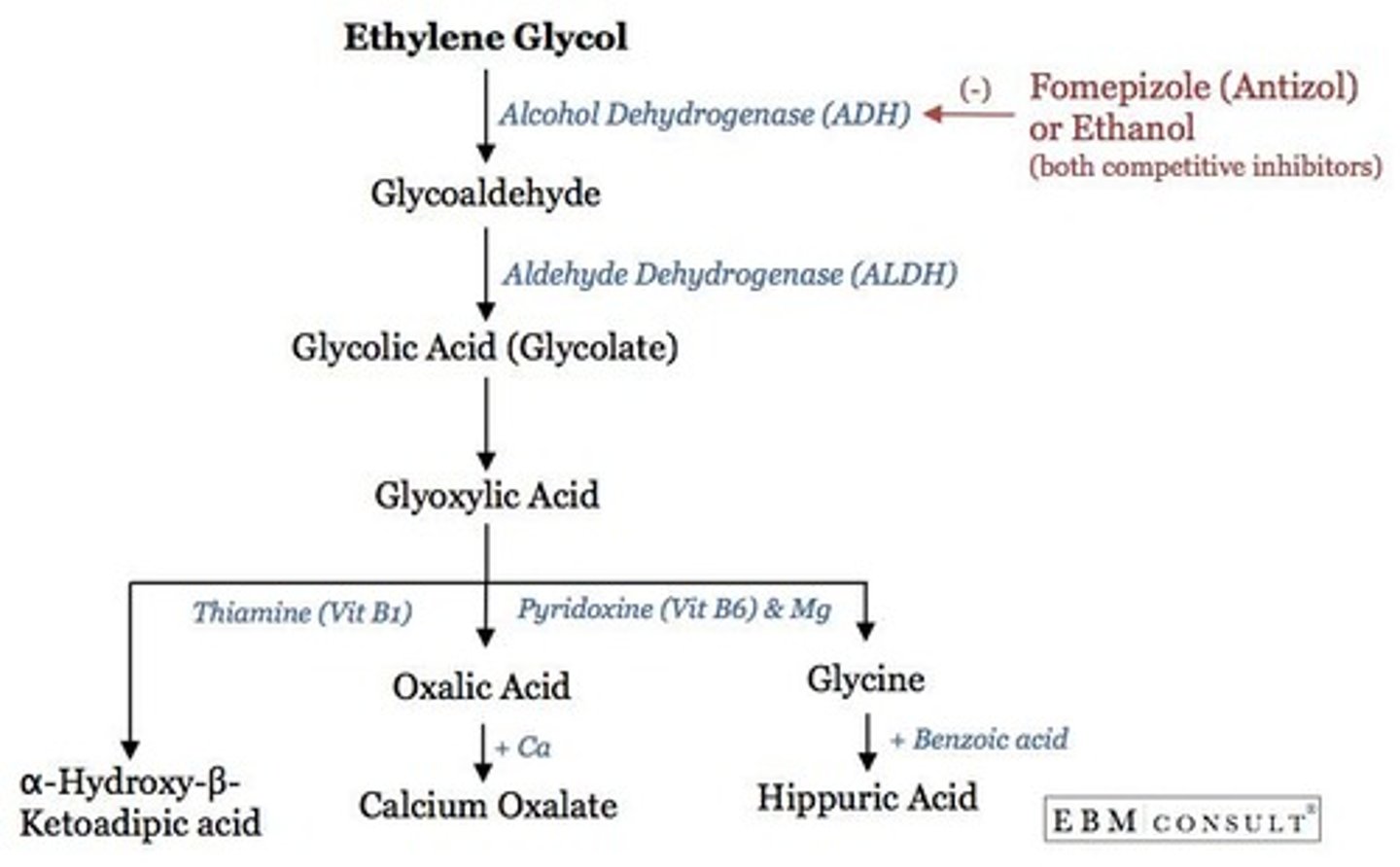
What is the effect of ethanol when used to prevent methanol poisoning?
Ethanol competes with methanol for the active site of alcohol dehydrogenase, reducing methanol's toxic effects.
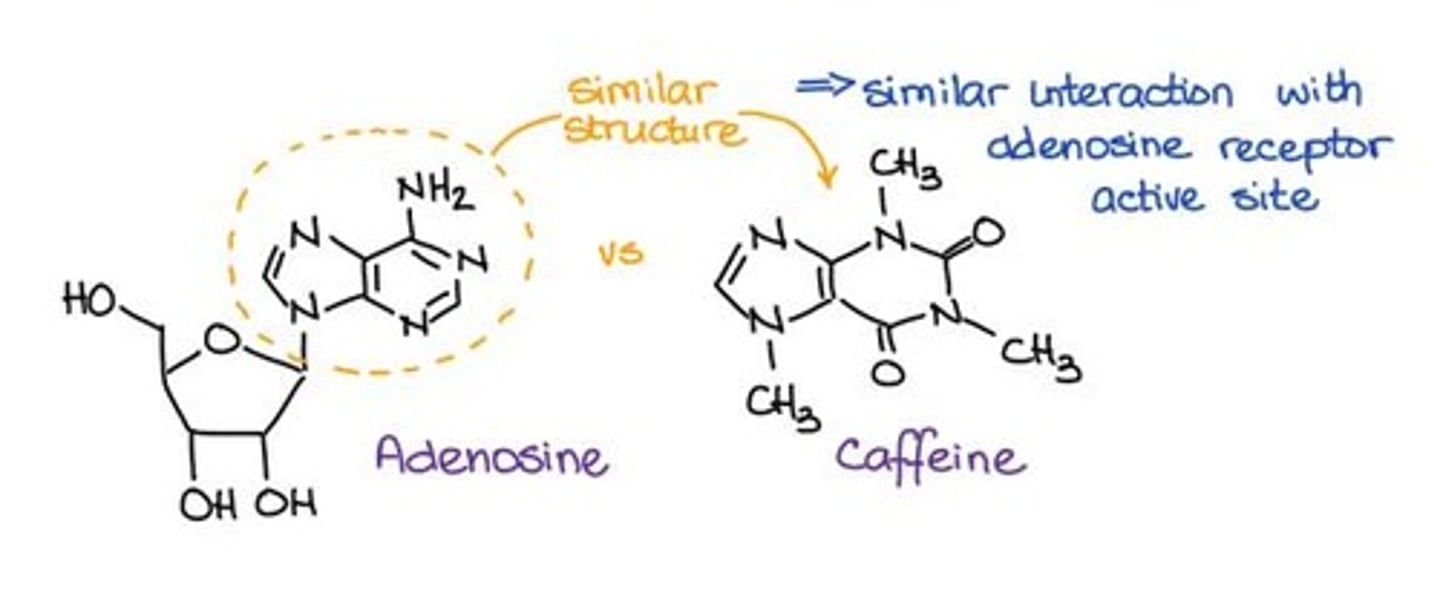
What role does caffeine play in relation to adenosine receptors?
Caffeine prevents adenosine from binding to its receptors, reducing fatigue.
What characterizes non-competitive inhibition?
The inhibitor binds to a different site than the substrate, affecting both free enzymes and enzyme-substrate complexes.
How does non-competitive inhibition affect Km and Vmax?
Km remains unchanged; Vmax decreases.
What is the effect of heavy metals on enzyme activity?
Heavy metals can bind to enzymes, altering their 3D shape and reducing their activity.
What is the impact of cyanide on cytochrome c oxidase?
Cyanide inhibits cytochrome c oxidase, halting ATP production in the electron transport chain.

What defines mixed inhibition?
The inhibitor can bind to either the free enzyme or the enzyme-substrate complex, but with different affinities.
How does mixed inhibition affect Km and Vmax?
Km can either increase or decrease depending on the binding affinity; Vmax decreases.
What is uncompetitive inhibition?
The inhibitor binds only to the enzyme-substrate complex, preventing product formation.
How do Km and Vmax change in uncompetitive inhibition?
Km decreases; Vmax decreases.
What is the significance of inositol monophosphatase in mood disorders?
Lithium binds to IMPase, preventing the release of inositol, which is crucial for mood regulation.
What happens when competitive levels of ethanol are administered in high methanol poisoning?
It can lead to unexpected results due to the high levels of methanol present.
What is Fomepizole used for?
Fomepizole is a compound that binds to methanol and alcohol dehydrogenase, reducing the production of toxic metabolites.
What is irreversible inhibition?
Irreversible inhibition, also known as enzyme inactivation or suicide inhibition, occurs when inhibitors form covalent bonds with key amino acids in target enzymes, leading to permanent inactivation.
What happens to enzyme activity after irreversible inhibition?
Once an enzyme is irreversibly inhibited, its activity cannot be restored by simply removing the inhibitor; new enzyme synthesis is required.
How can irreversible inhibitors be used in research?
They can identify functional amino acids within an enzyme's active site by measuring the loss of activity after treatment with a residue-specific irreversible inhibitor.
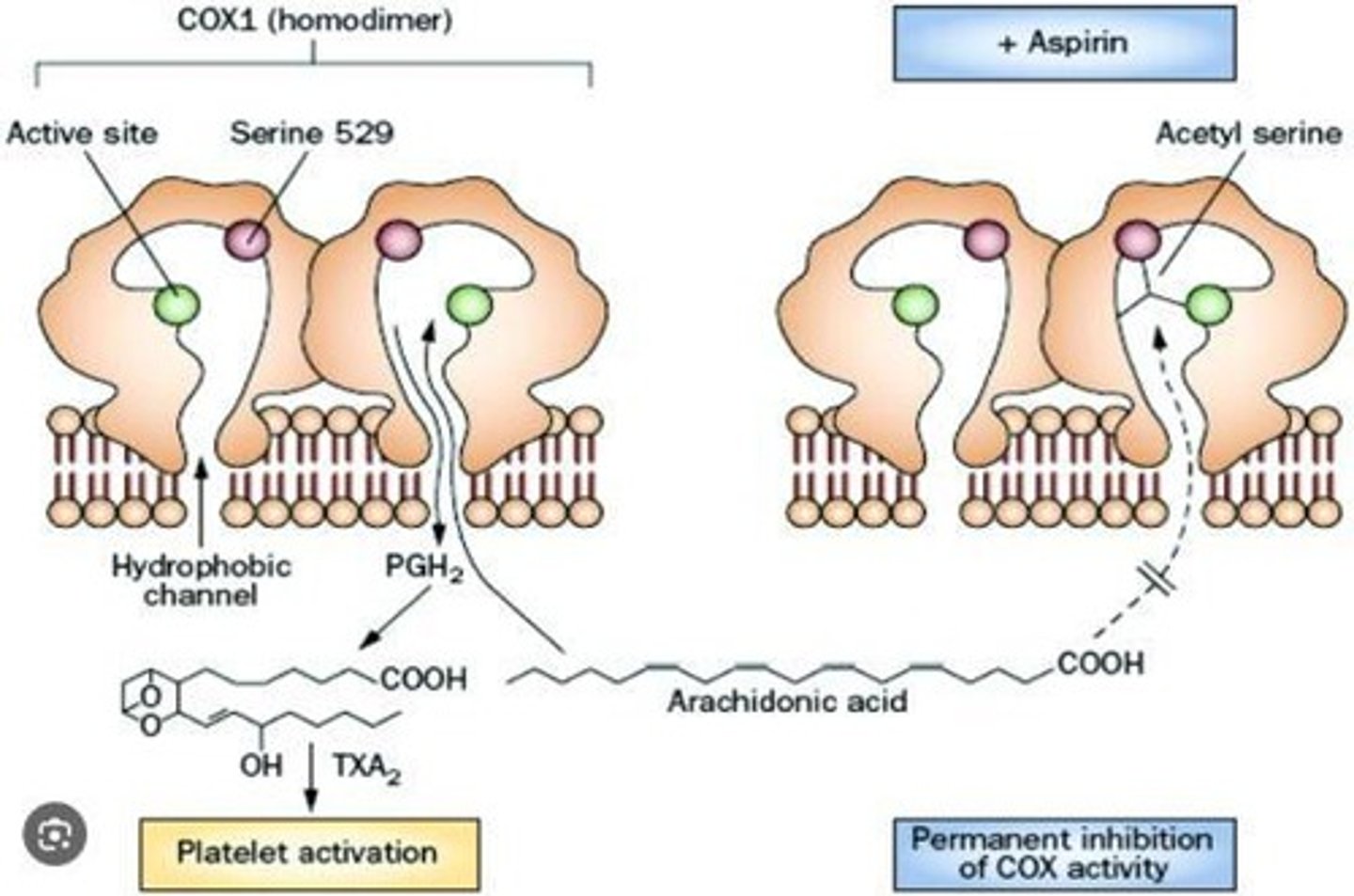
What role does arachidonic acid play in the body?
Arachidonic acid serves as a substrate for cyclooxygenase (COX) enzymes, which convert it to prostaglandins, mediators of pain and inflammation.
How does aspirin function as an irreversible inhibitor?
Aspirin competes with arachidonic acid for the active site of COX enzymes, preventing the sensations of pain associated with their activity.
What is African trypanosomiasis and how is it treated?
African trypanosomiasis, or sleeping sickness, is treated with Eflornithine, a suicide inhibitor that irreversibly binds to ornithine decarboxylase, reducing polyamine production necessary for parasite growth.
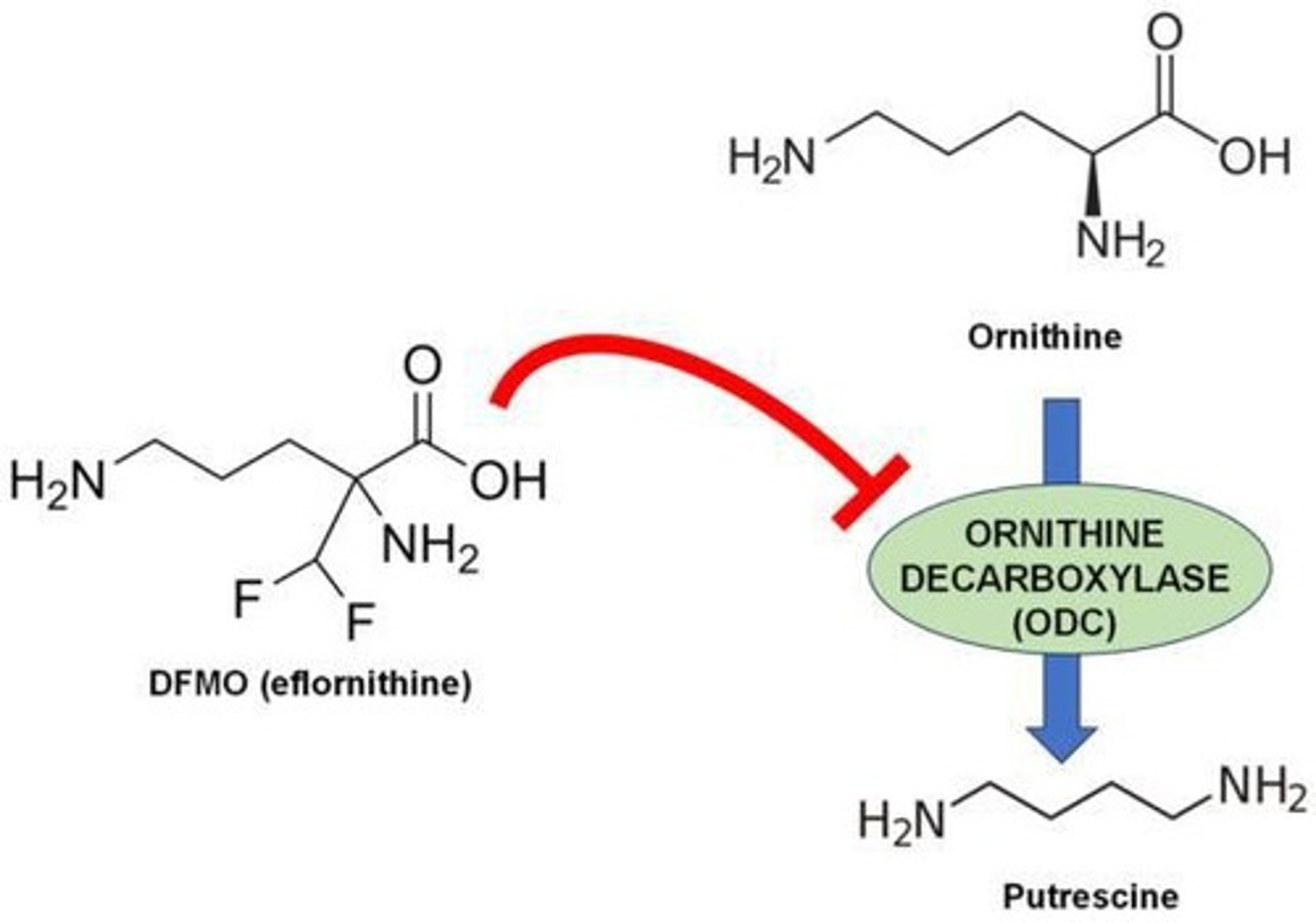
What is the mechanism of action of penicillin?
Penicillin is an irreversible inhibitor of bacterial cell wall synthesis, covalently modifying glycopeptide transpeptidase to stop its catalytic activity.
What is beta-lactamase and how does it affect antibiotic efficacy?
Beta-lactamase is an enzyme produced by some bacteria that degrades antibiotics containing a beta-lactam ring, leading to antibiotic resistance.
What is the role of clavulanic acid in antibiotic treatment?
Clavulanic acid is a suicide inhibitor that irreversibly inhibits beta-lactamase, often used in combination with beta-lactam antibiotics to combat resistant bacterial strains.
What are the three main classes of receptors defined by their transduction mechanism?
Ion-Channel-linked, G-Protein-linked, and Enzyme-linked Receptors.
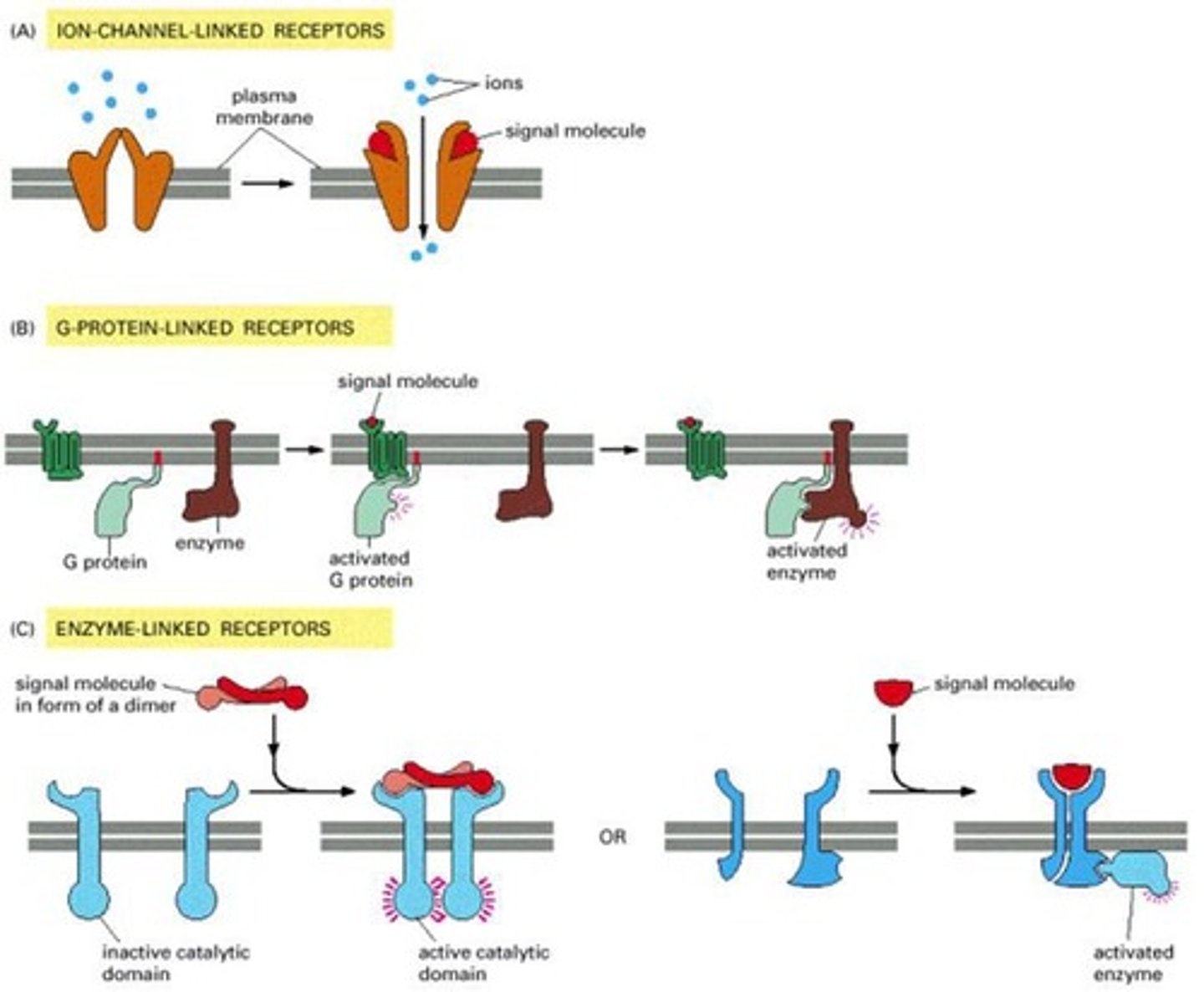
How do cell-surface receptor proteins function?
They act as signal transducers, converting extracellular ligand-binding events into intracellular signals that alter the behavior of the target cell.
What is the significance of phosphorylation in cell signaling?
Phosphorylation is crucial for activating or inactivating proteins, thereby regulating various cellular processes.
What are ion channels and their role in cell signaling?
Ion channels are transmembrane proteins that transport inorganic ions and are gated, opening in response to specific stimuli, affecting the excitability of cells.
Ion Channel Mechanism
What distinguishes voltage-gated ion channels from ligand-gated ion channels?
Voltage-gated channels open in response to changes in membrane potential, while ligand-gated channels open upon binding of a neurotransmitter.
What role do voltage-gated Ca2+ channels play in cellular signaling?
They allow Ca2+ entry into the cytoplasm, acting as a second messenger and initiating various cellular events such as muscle contraction and hormone secretion. - ADD MORE LATER
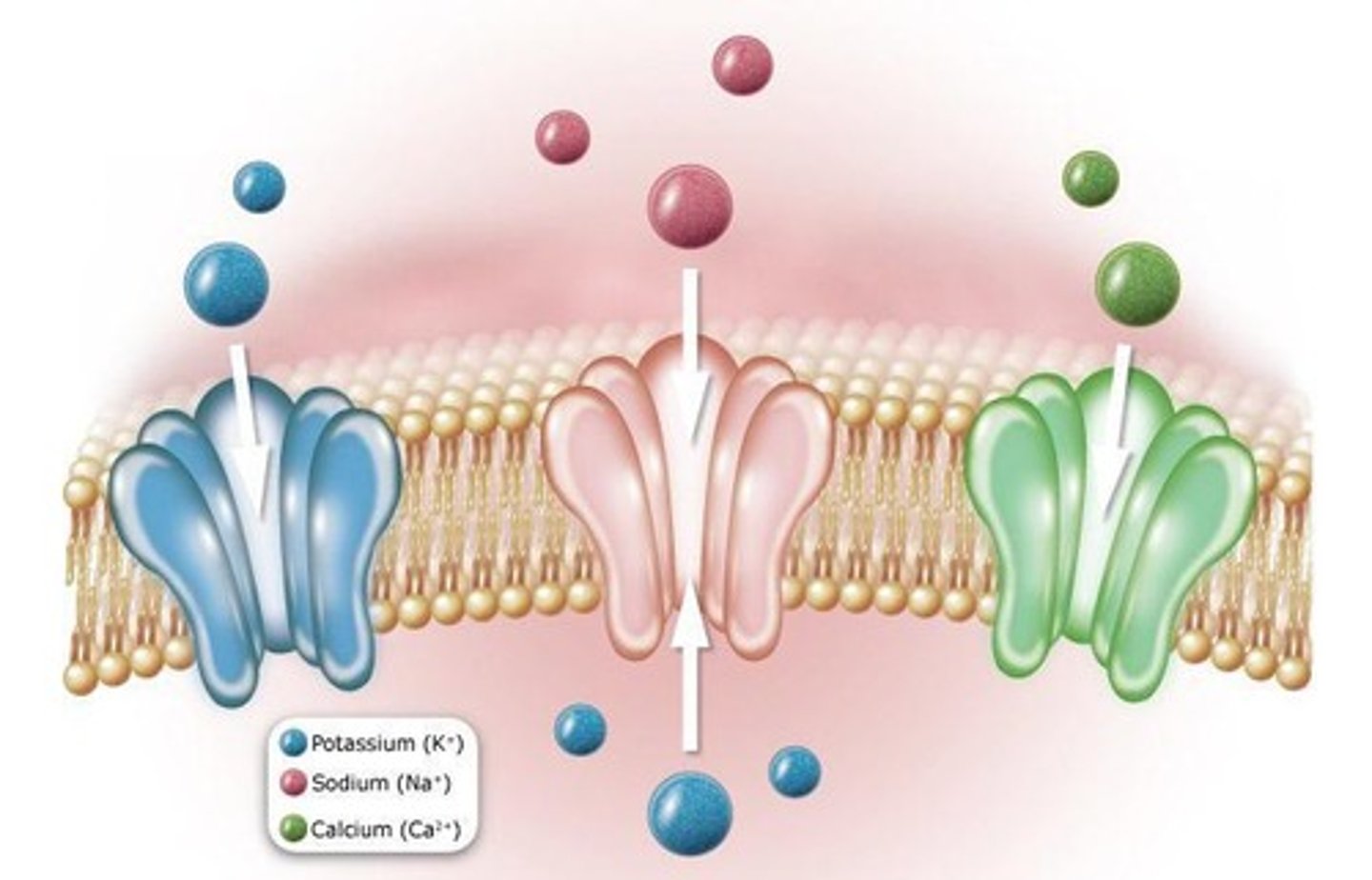
What is channelopathy?
Channelopathy refers to disorders caused by defects in ion channel function, including diseases resulting from mutations or autoimmune attacks.
What are enzyme-linked receptors and their primary functions?
Enzyme-linked receptors are transmembrane proteins that promote processes like cell proliferation and differentiation upon ligand recognition.
where are the binding sites located in enzyme linked receptors?
They are single-pass transmembrane proteins, their ligand-binding site outside the cell and their catalytic or enzyme-binding site inside.
How do receptor tyrosine kinases (RTKs) function?
- RTKs have intrinsic kinase activity, autophosphorylating tyrosine residues upon ligand binding, which serves as docking sites for downstream signaling molecules.
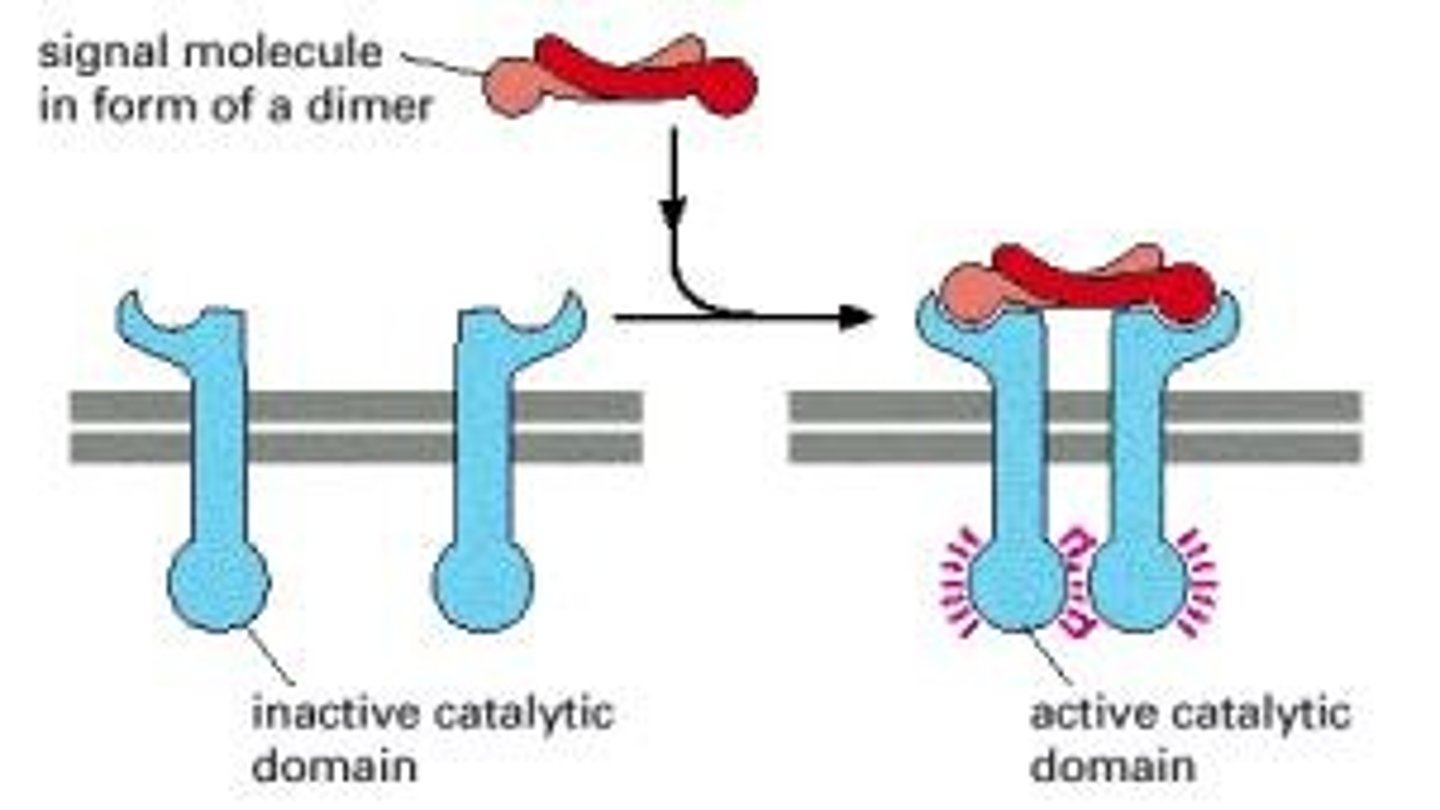
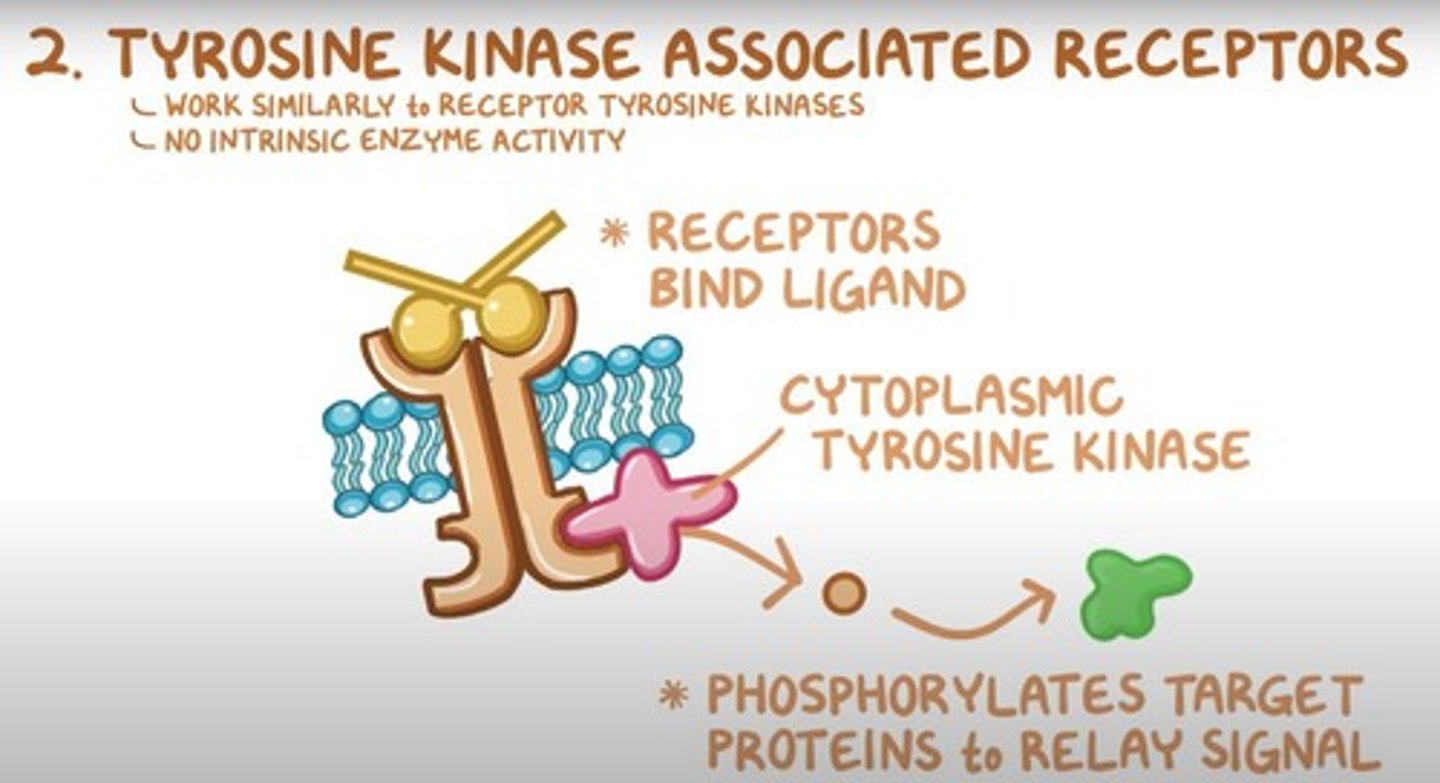
What is the role of tyrosine kinase-associated receptors (TKARs)?
TKARs do not have intrinsic kinase activity but activate associated cytoplasmic tyrosine kinases upon ligand binding.
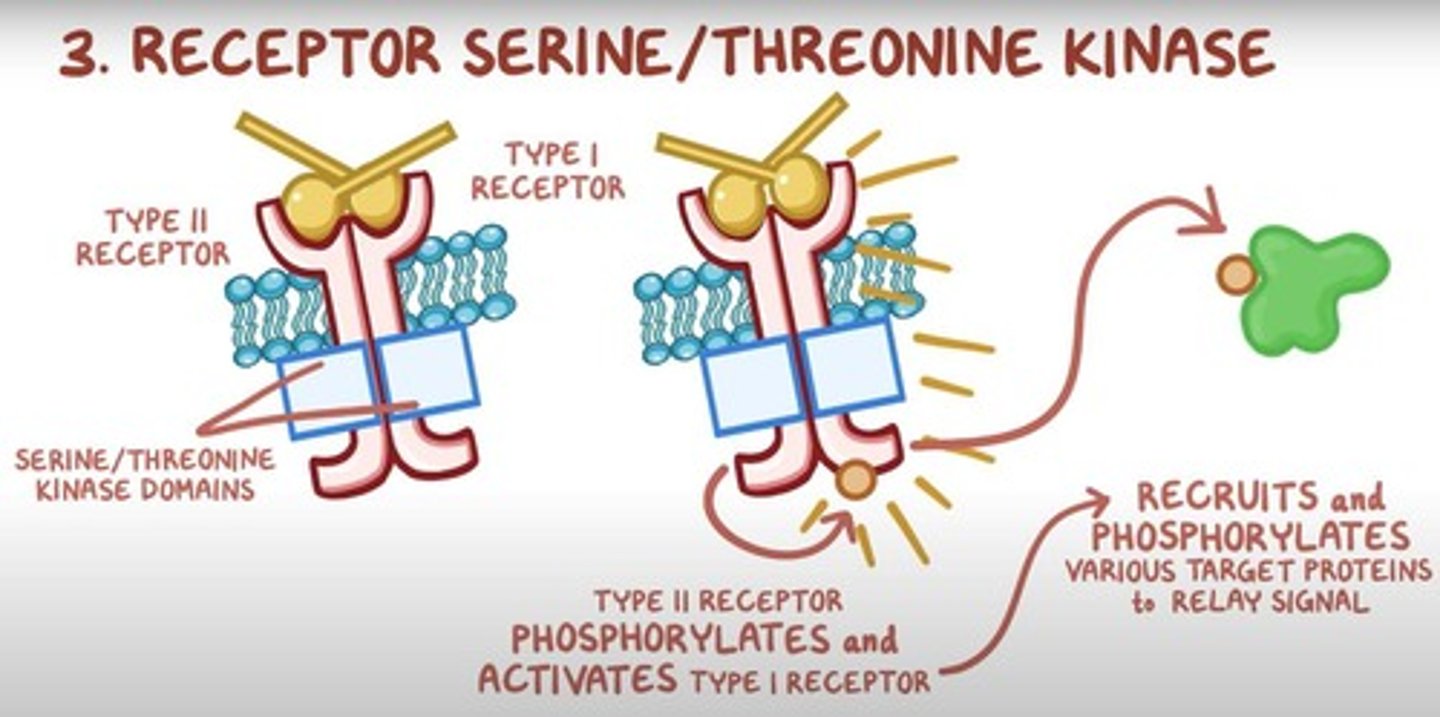
What are serine/threonine kinase receptors and their function?
These receptors activate intracellular serine/threonine kinases in response to ligand binding, initiating signaling pathways through phosphorylation.
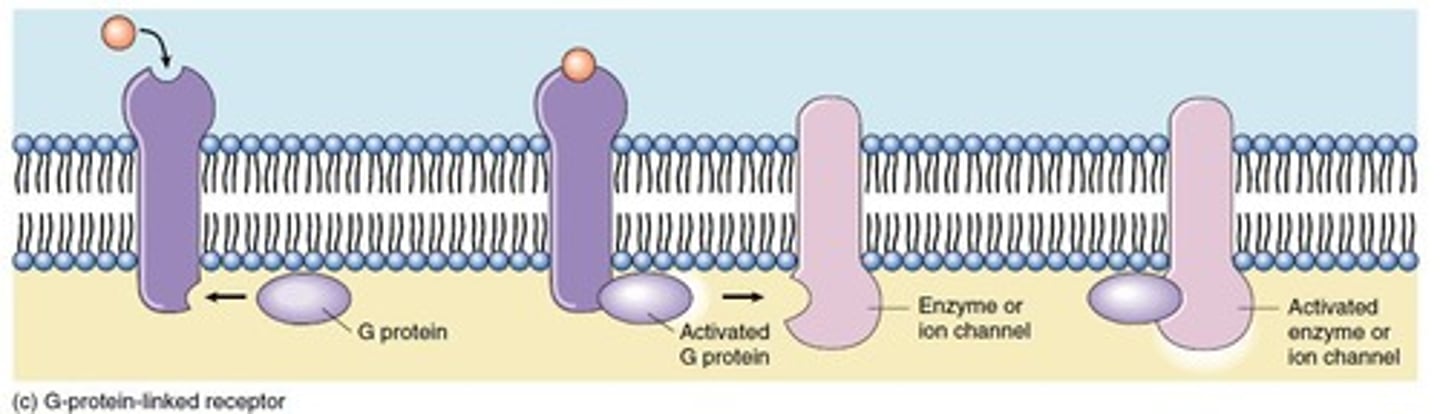
What is the largest family of cell surface receptors?
G-Protein-Coupled Receptors (GPCRs) are the largest family, mediating interactions with trimeric G proteins.
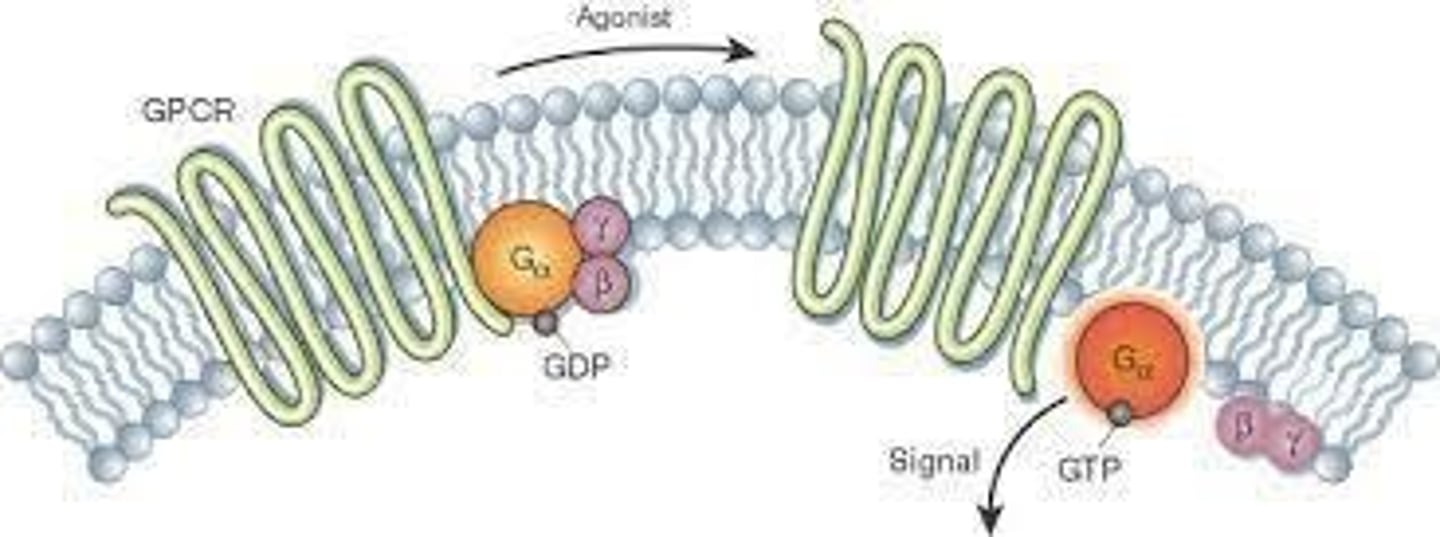
Describe the step-by-step mechanism of GPCR signaling.
1. Signal Binding - A ligand binds to a GPCR on the cell surface.
2. Activation of G Protein - The GPCR undergoes a conformational change and activates the G protein by
promoting the exchange of GDP for GTP on the α-subunit.
3. Subunit Dissociation - The GTP-bound α-subunit dissociates from the βγ dimer.
4. Effector Activation - These subunits activate or inhibit target enzymes
5. Cellular Response - Second messengers activate
downstream signalling pathways.
6. Termination - The α-subunit hydrolyses GTP to GDP , inactivating itself. The α, β, and γ subunits reassociate, returning the G protein to its inactive state.
What are second messengers in cell signaling?
Second messengers are small intracellular signaling molecules generated in response to receptor activation, broadcasting signals within the cell.
How do second messengers like cyclic AMP and Ca2+ function?
They diffuse rapidly within the cell, binding to and altering the behavior of selected signaling proteins or target proteins.
What is the role of G proteins in GPCR signaling?
G proteins mediate the interaction between GPCRs and target proteins, affecting intracellular mediators and ion permeability.
What is the difference between primary and secondary responses in cell signaling?
Primary responses are immediate effects of signaling, while secondary responses are delayed effects that often involve changes in gene expression.
How do cells respond to internal and external stimuli?
Cells respond by altering their behavior through signaling pathways that modify gene expression, enzyme activity, or cytoskeletal arrangements.
What are molecular switches in cell signaling?
Molecular switches are proteins that toggle between active and inactive states in response to signals, crucial for signal transmission.
How does signal amplification occur in the cell?
Signal amplification occurs through signaling cascades where each step activates multiple downstream proteins, enhancing the overall response.
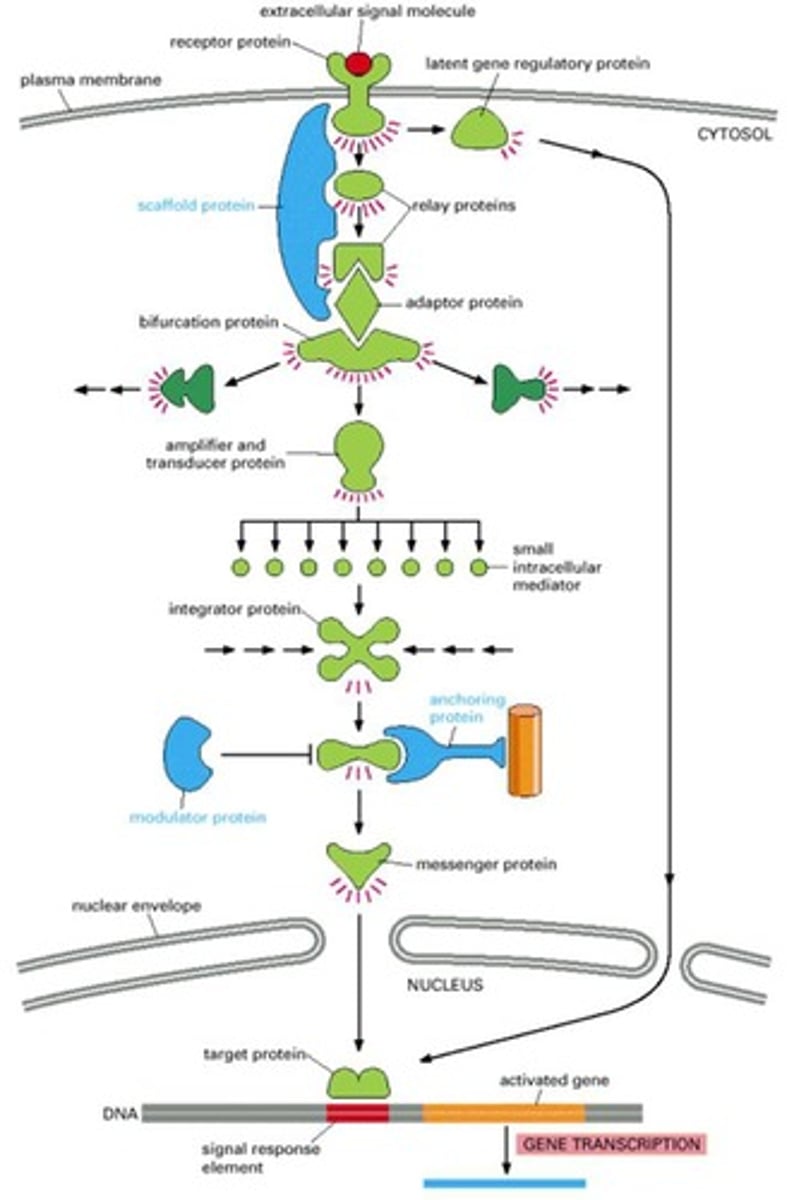
What are relay proteins in cell signaling?
Relay proteins transmit the signal to the next component in the signaling pathway without altering the signal.
What is the function of messenger proteins?
Messenger proteins transport signals from one part of the cell to another, such as from the cytosol to the nucleus.
What do adaptor proteins do in signaling pathways?
Adaptor proteins connect different signaling proteins without conveying a signal themselves.
What are amplifier proteins and their role?
Amplifier proteins, often enzymes or ion channels, significantly increase the signal by producing large amounts of mediators or activating many downstream proteins.
What is the role of transducer proteins in signaling?
Transducer proteins convert signals into different forms, such as the enzyme that produces cyclic AMP.
What do anchoring proteins do?
Anchoring proteins tether signaling proteins to specific locations within the cell, maintaining their spatial organization.
What are effector/target proteins?
Effector proteins execute specific cellular responses, including changes in gene expression and enzyme activity.
What is the function of integrator proteins?
Integrator proteins coordinate and regulate cellular responses to multiple signals from various pathways.
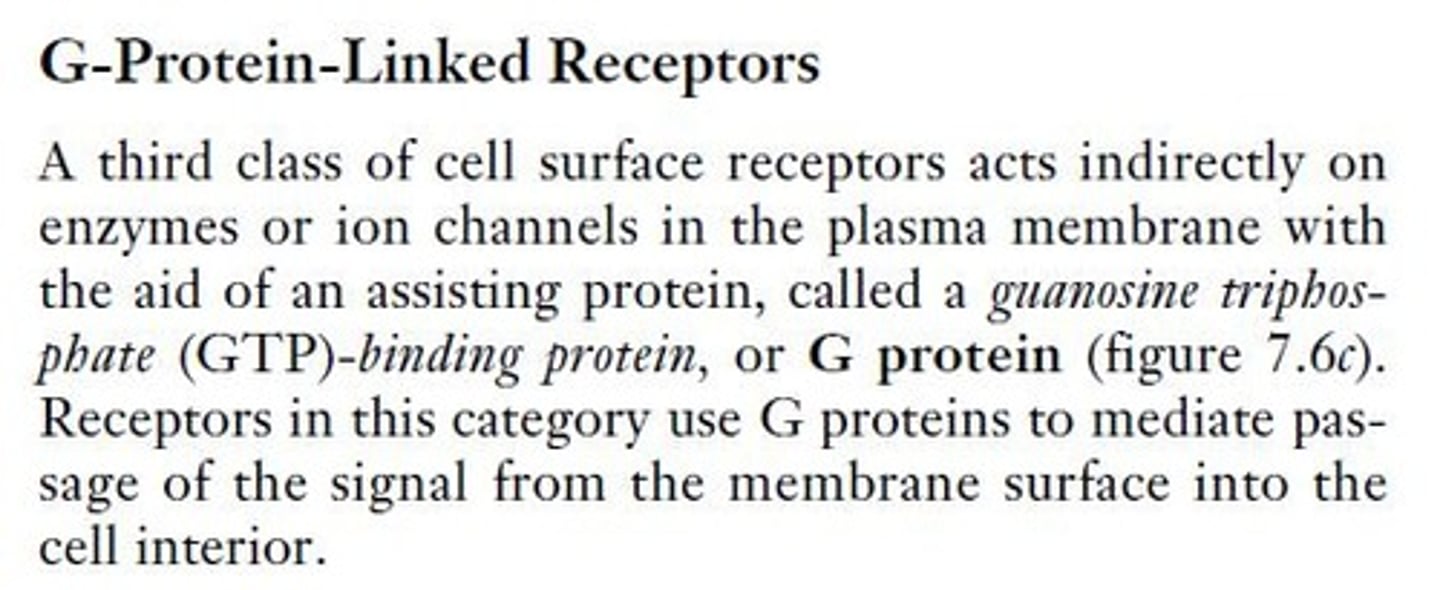
How does phosphorylation affect protein activity?
Phosphorylation can activate or inactivate proteins by adding or removing phosphate groups.
What are the two main types of protein kinases?
The two main types are serine/threonine kinases, which phosphorylate serines and threonines, and tyrosine kinases, which phosphorylate tyrosines.
What percentage of genes encode protein kinases in humans?
Approximately 2% of human genes encode protein kinases.
What is a phosphorylation cascade?
A phosphorylation cascade is a series of events where one protein kinase activates another, amplifying the signal as it progresses.
What is the MAPK signaling cascade?
The MAPK signaling cascade is a pathway that involves a series of kinases, including Raf, activated by receptors to transmit signals.
What is the role of Raf in the MAPK cascade?
Raf phosphorylates and activates MEK
What does MEK do in the MAPK cascade?
MEK phosphorylates and activates the ERKs.
What do ERKs phosphorylate and activate?
A variety of target molecules, including transcription factors like c-Myc.
What is the outcome of the activation of targets by ERKs?
They promote cell growth and division.
What is a mitogen-activated protein kinase (MAPK) cascade?
A three-tiered kinase signaling pathway involving Raf, MEK, and ERKs.
What is a mitogen?
A signal that causes cells to undergo mitosis or divide.
What are proto-oncogenes?
Genes encoding proteins like growth factor receptors, Raf, and c-Myc that, when overactive, are associated with cancer.
Which amino acids can phosphate groups be attached to?
Tyrosine, threonine, and serine.
What is the function of kinases in cell signaling?
Kinases catalyze the transfer of phosphate groups to target proteins.
What role do phosphatases play in cell signaling?
Phosphatases remove phosphate groups from proteins, reversing phosphorylation.
What are the two major types of GTP-binding proteins?
Large trimeric GTP-binding proteins (G proteins) and small monomeric GTPases.
What initiates primary cell signaling?
The activation of cell surface receptors by extracellular signaling molecules.
What is signal transduction?
The process triggered by receptor activation that leads to intracellular events.
How quickly does the primary response occur after receptor activation?
Within about 30 minutes.
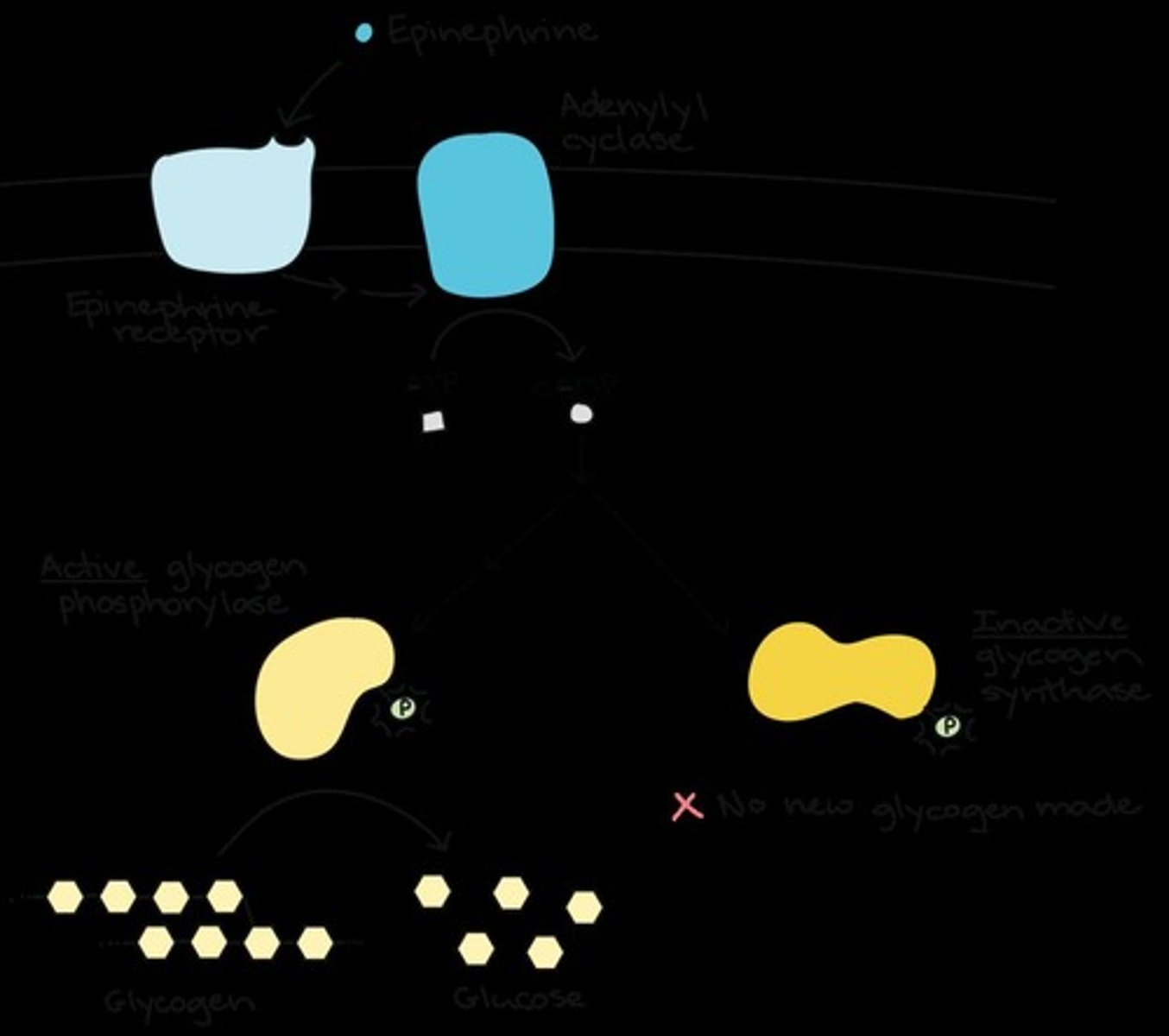
What can a single epinephrine ligand trigger in terms of glucose production?
It can elicit the production of hundreds of millions of glucose molecules.