Grignard Rxn- Syenthesis of a Benzoic Acid Derivative
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
14 Terms
how is a grignard reagent created ?
u start w an organohalide (partial + and -) which has the electrophilic carbon
what does adding Mg do to your organohalide?
the magnesium attaches b/w the R group and halide making the R group hold the partial negative and Mg hold the partial positive
what kind of mechanism is forming the grignard reagent ?
a radical mechanism
Grignard reagent are both…
nucleophilic and basic
how do grignard reagents attach to carbonyl carbons?
it attacks it making the double bond add as lone pairs on the oxygen giving it a negative charge
how do u rid of the negative charge on the O ?
u add H3O+ and the lone pairs on the oxygen attack an H creating an alcohol group
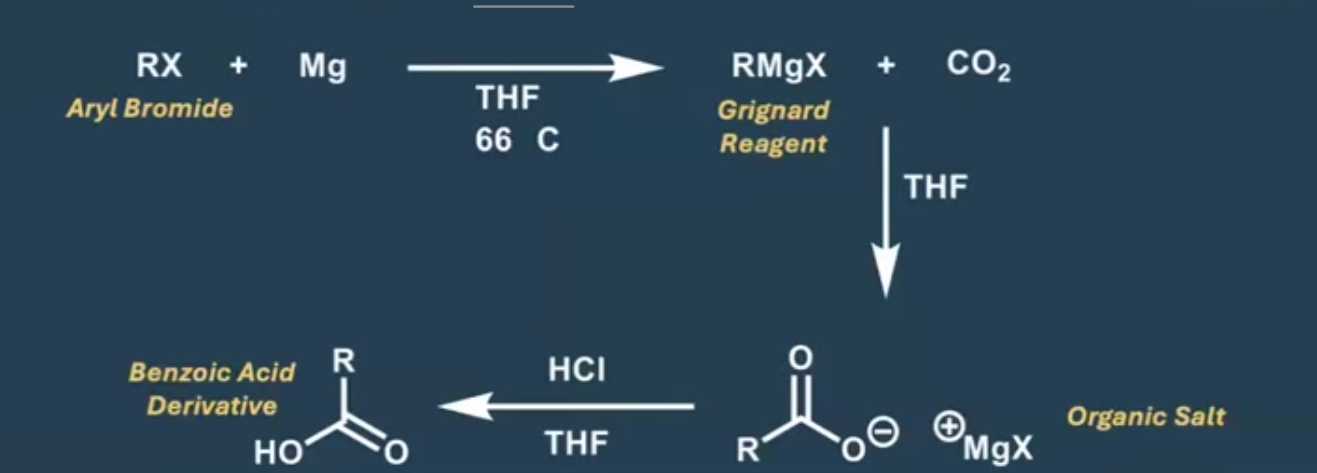
what was the starting material for the Benzoic Acid Derivative Rxn?
CO2 solid
what’s the first step of the benzoic acid derivative rxn?
the bond bw the R group and grignard reagent attacks the C in the CO2, making the bond go onto an O as a l.p. creating negative charge
what does the intermediate now have after the first step?
a new C-C bond
how is the carbolic acid formed ?
the O- attacks the H on the H3O+ also making H2O
how many arrows are in the benzoic acid derivative mechanism?
4 w/2 in each step
using a grignard reagent as a base results in…
it bonding to an H instead of having a nucleophilic carbon which is why our glassware had to be in the oven to dry
what were the steps done in the benzoic acid derivative experiment ?
synthesis
extraction
isolation
MP, IR, TLC, and NMR
benzoic acid derivative mechanism