Orgo 2 Exam 1
1/148
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
149 Terms
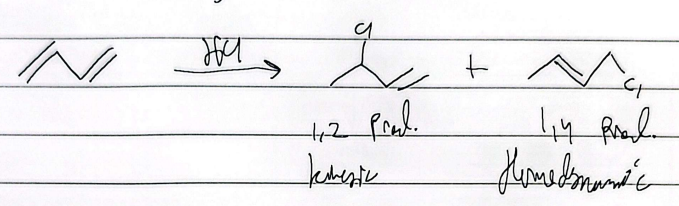
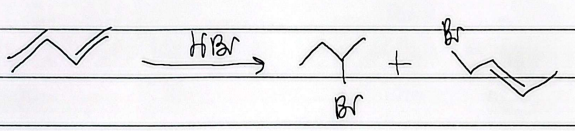
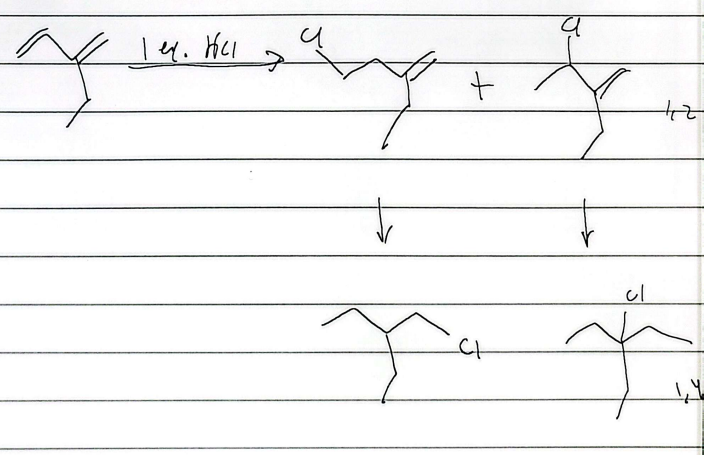
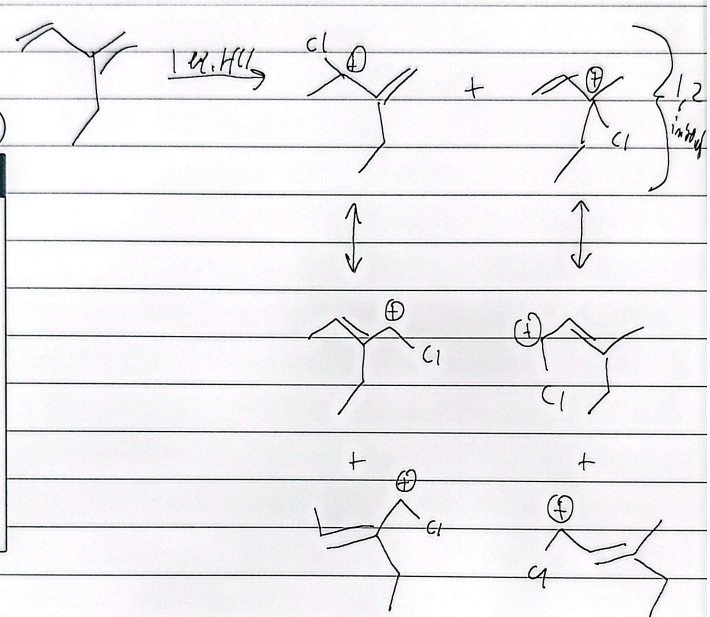
hydrohalogenation
mark

hydrohalogenation
hydrohalogenation
hydrohalogenation
hydrohalogenation
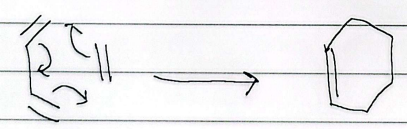
diels alder
s-cis
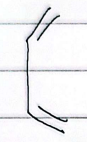
s-trans

diels alder
diels alder
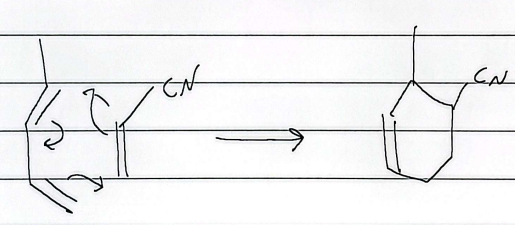
diels alder
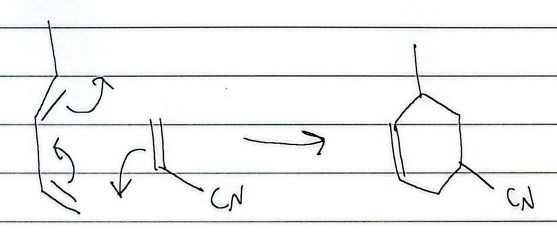
endo rule
substituents must be endo while the hydrogens must be exo
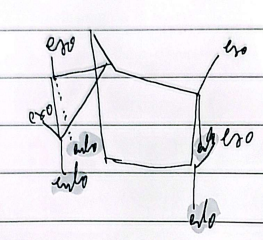
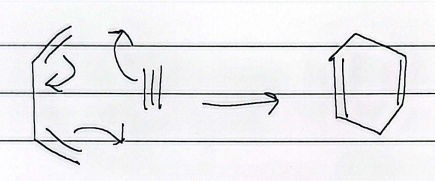
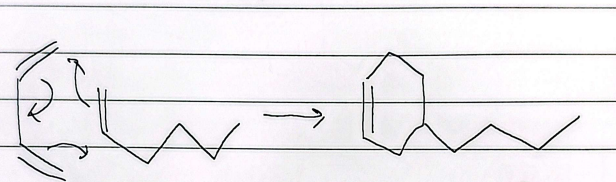
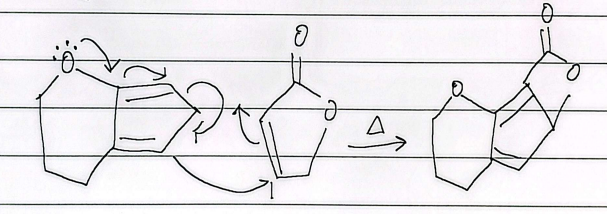
bicyclic diels alder
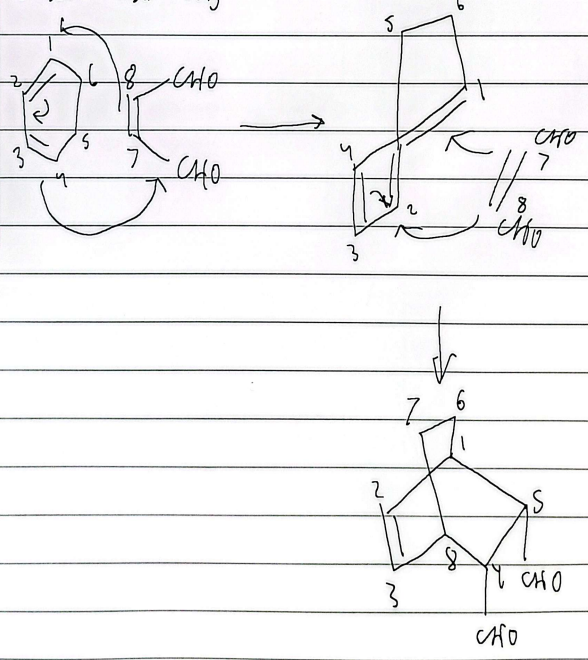
bicyclic diels alder
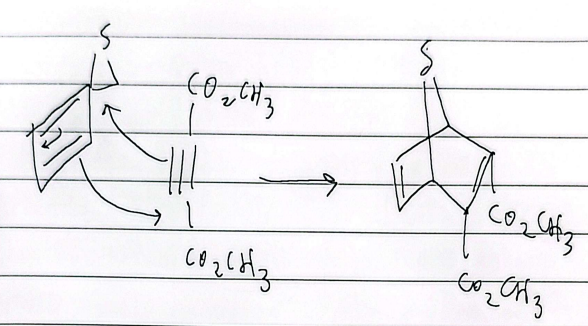
retro diels alder
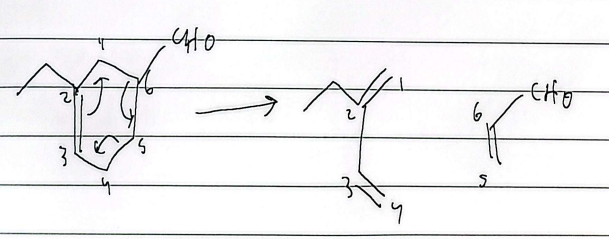
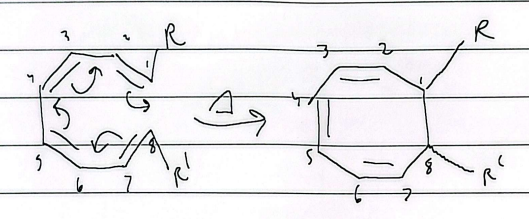
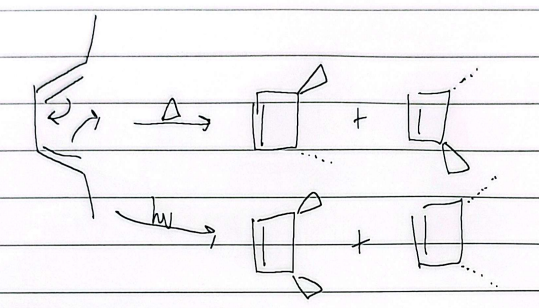
electrocyclic
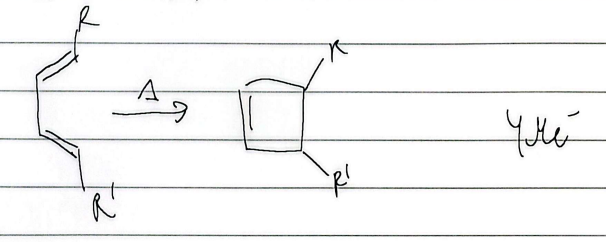
electrocyclic
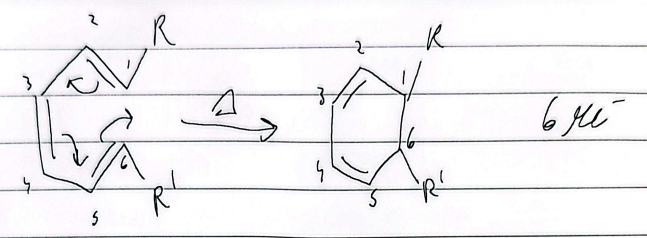
electrocyclic
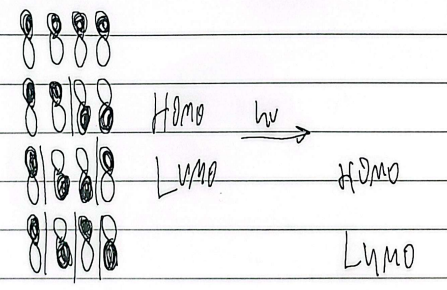
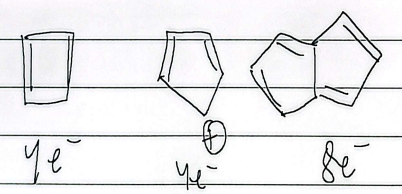
4e-
heat trans, light cis
4e-
heat trans, light cis
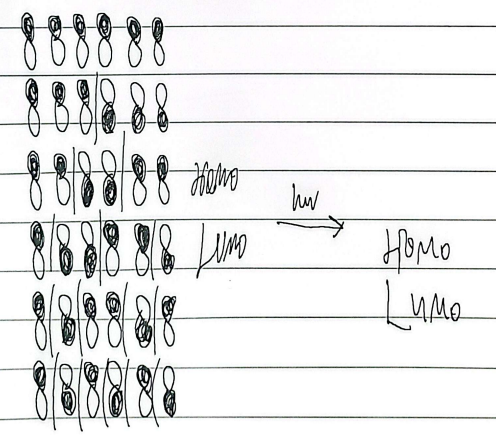
6e-
heat cis, light trans
6e-
heat cis, light trans
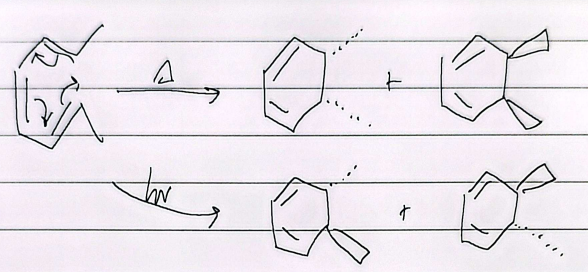
8e-
heat trans, light cis
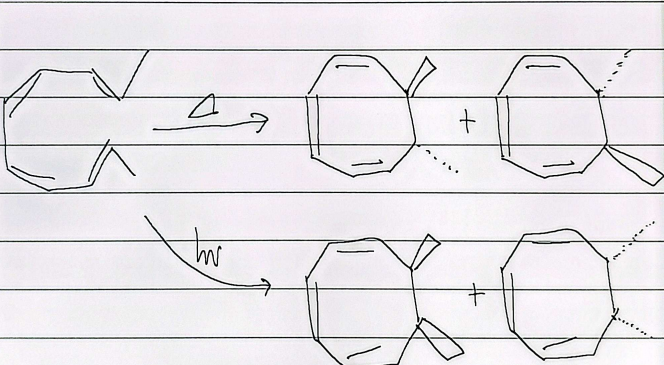
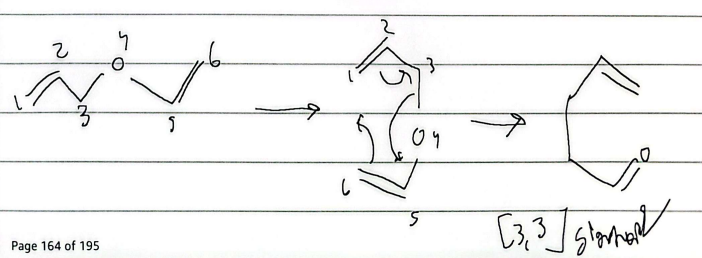
sigmatropic rearrangement
when symmetrical, 3-3 is formed
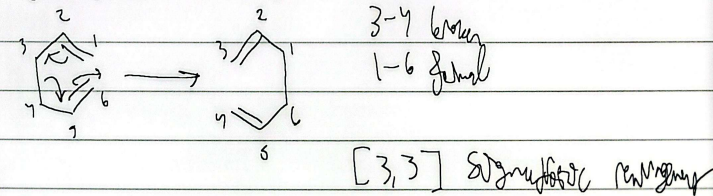
sigmatropic rearrangement

sigmatropic rearrangement
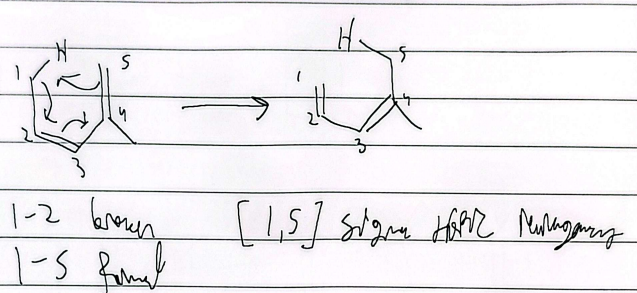
allyl vinyl ether
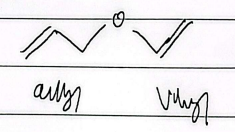
aryl vinyl ether
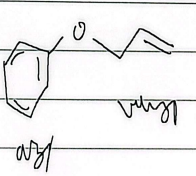
sigmatropic rearrangement
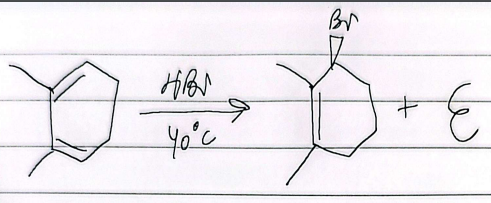
hydrohalogenation
diels alder
bicyclic diels alder
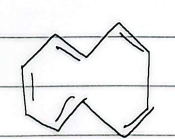
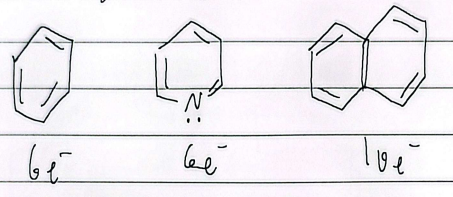
10 annulene
non-aromatic
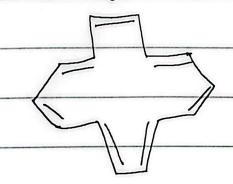
14 annulene
aromatic
2 annulene
antiaromatic
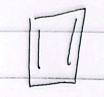
6 annulene
aromatic
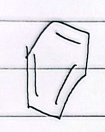
8 annulene
nonaromatic
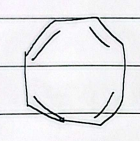
12 annulene
antiaromatic
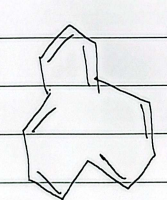
antiaromatic
a ring is ________ if it’s aromatic in every way except it’s number of pi e- is a multiple of 4 (4n; 4, 8, 12, 16..)
aromatic
planar, fully conjugated, delocalized e-, 4n+2 pi e-, all carbons must be sp2 to be planar
ortho
1,2
meta
1,3
para
1,4
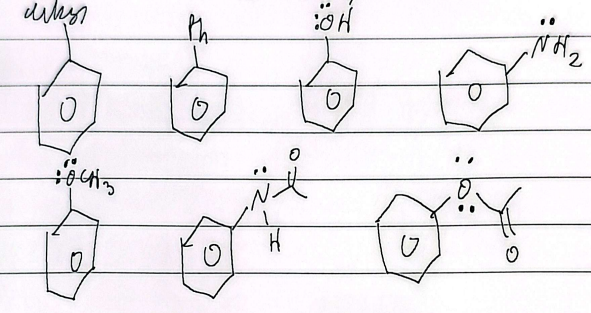
priority
aldehyde > ketone > alcohol > amine > alkyl
phenol
aniline
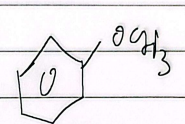
anisole
toluene

benzoic acid
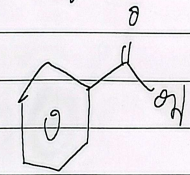
benzaldehyde
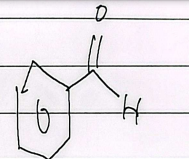
o-xylene
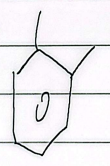
m-xylene
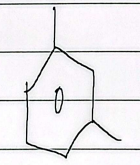
p-xylene
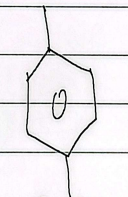
acetophenone
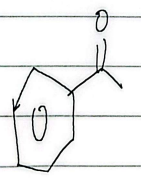
benzophenone
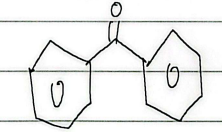
styrene
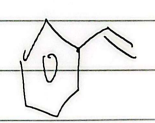
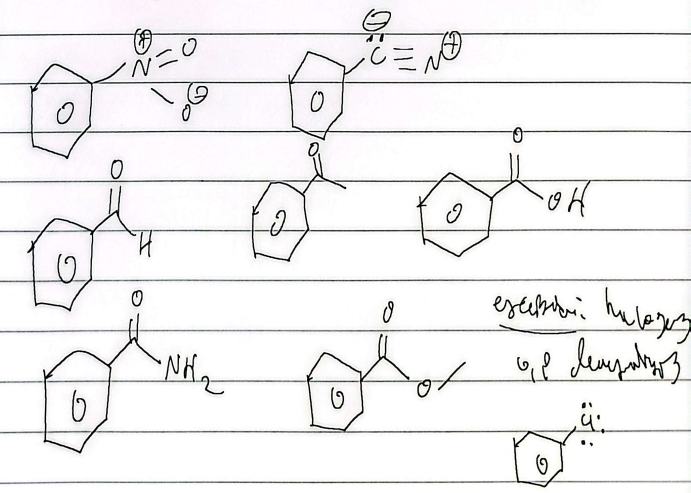
oxidation
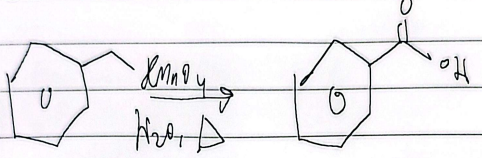
oxidation
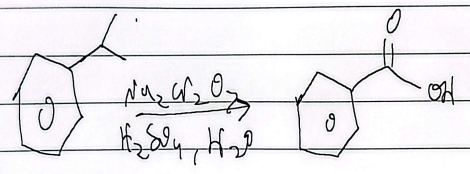
oxidation

free-radical halogenation
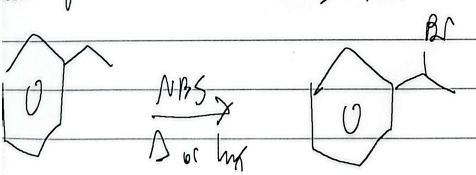
substitution

substitution
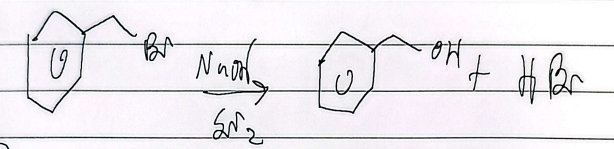
elimination

ellimination
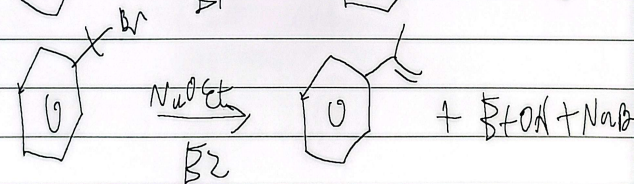
elimination
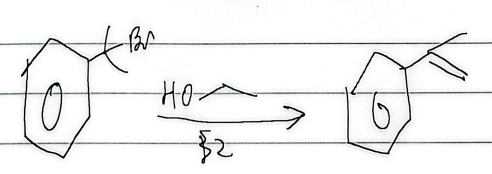
birch reduction
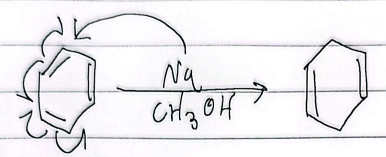
birch reduction
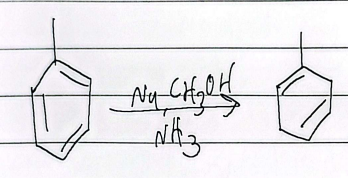
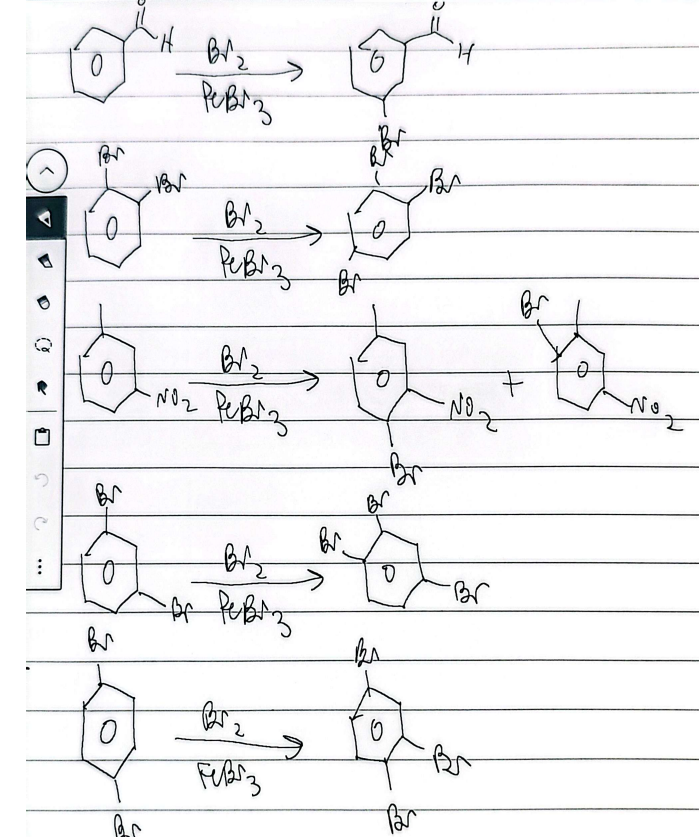
halogenation
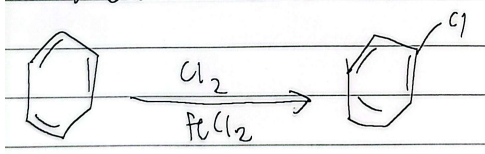
halogenation

EAS mechanism
E = Cl or Br
B: = FeCl4 or FeBr4
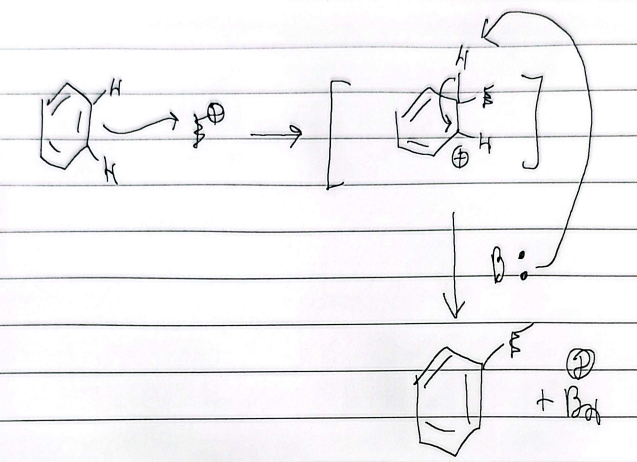
halogenation
selefluor is used

halogenation
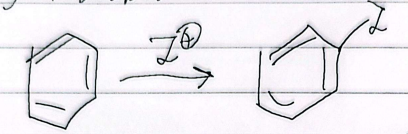
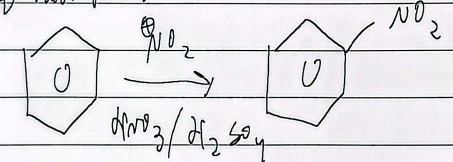
nitration
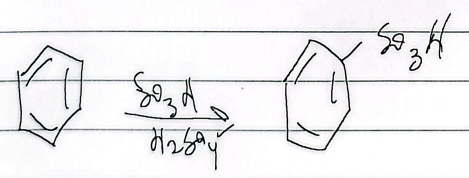
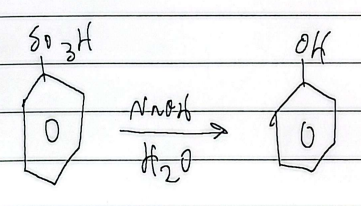
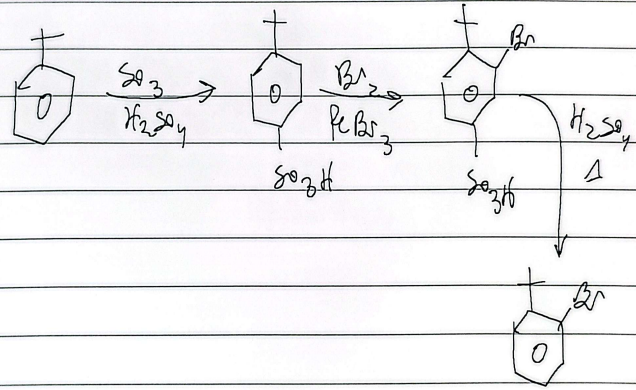
hydrosulfonation
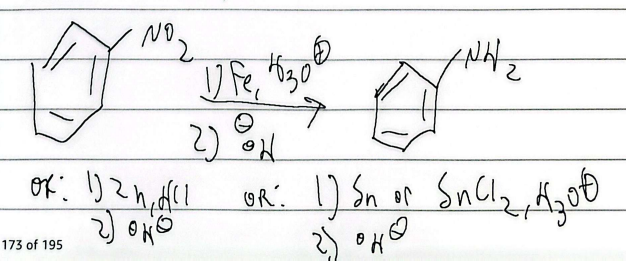
reduction
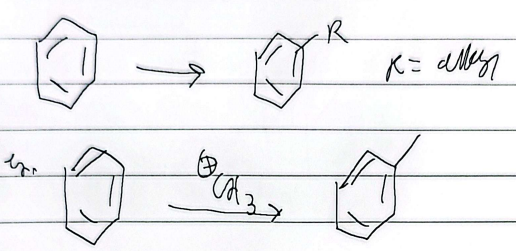
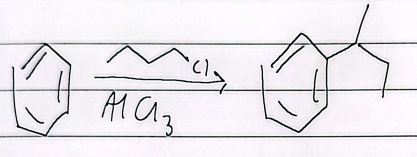
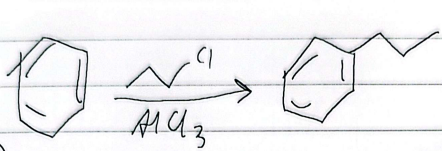
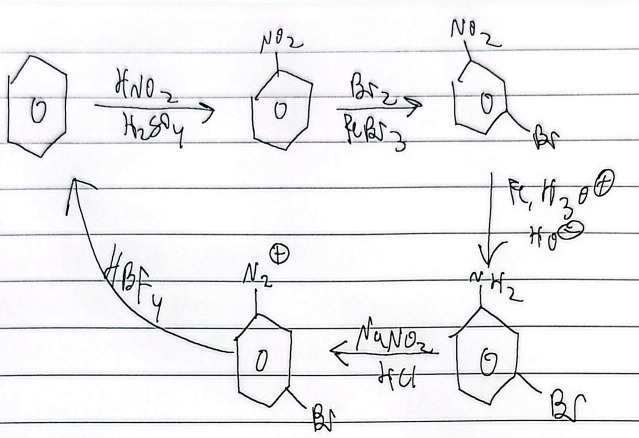
friedel-craffs alkylation
limitations
any alkyl halide can be used (single bonds only)
impossible to stop at mono-alkylation (multiple substitutions)
wont react if theres a strong deactivator
rearrangement
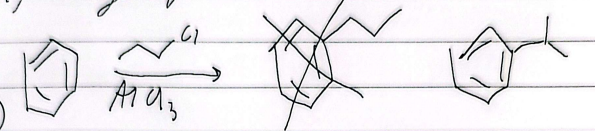
rearrangement
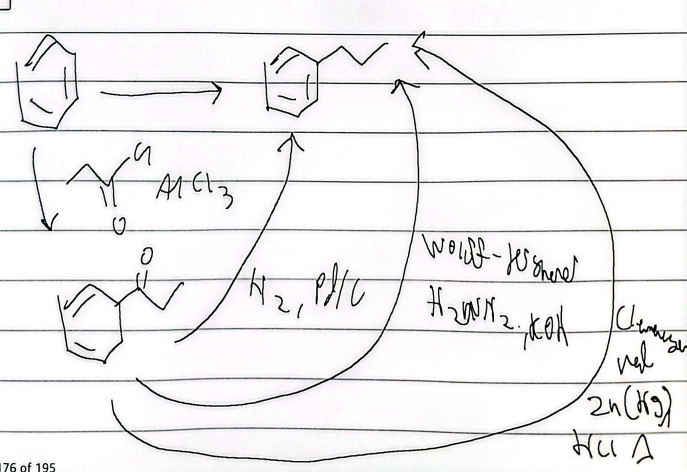
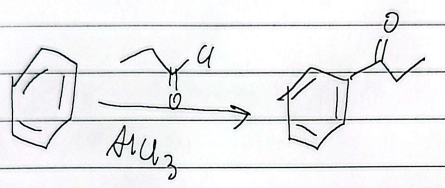
friedel-acylation
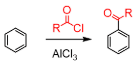
friedel-acylation
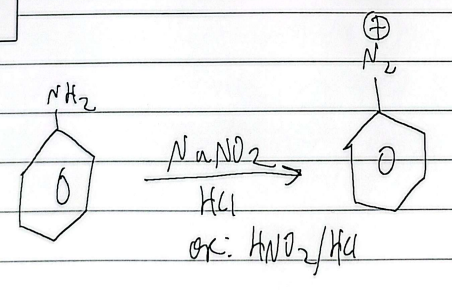
sandmeyer
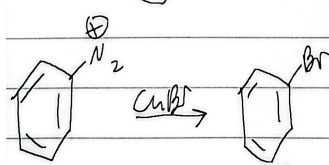
sandmeyer
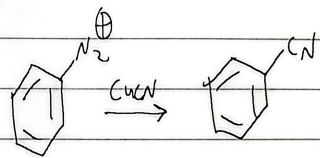
sandmeyer

rearrangement
friedel acylation
reduction
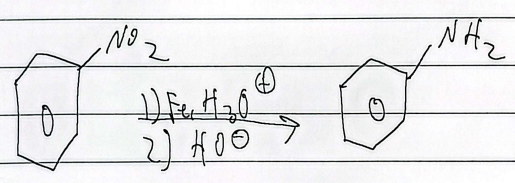
nh2 to n2+
aromatic rxns
hydrolysis
activating groups
faster rxn, lone pair e- directly connected to benzene, neg or partial neg charge
ortho/para director
deactivating groups
slower rxn, pos or partiall pos charge
meta director
exception: halogens ortho/para deactivators
halogenation
sulfonation and halogenation
chlorination
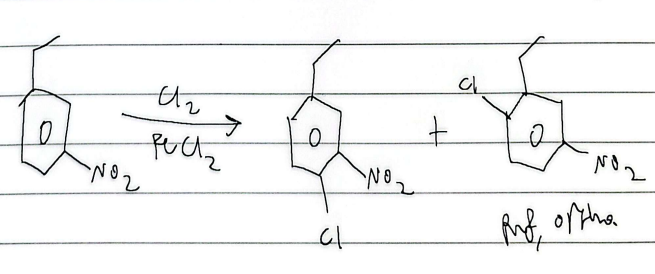
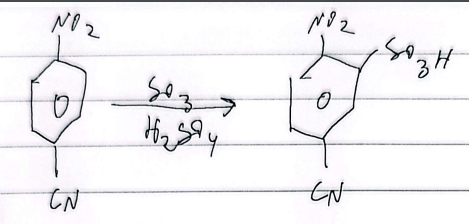
sulfonation
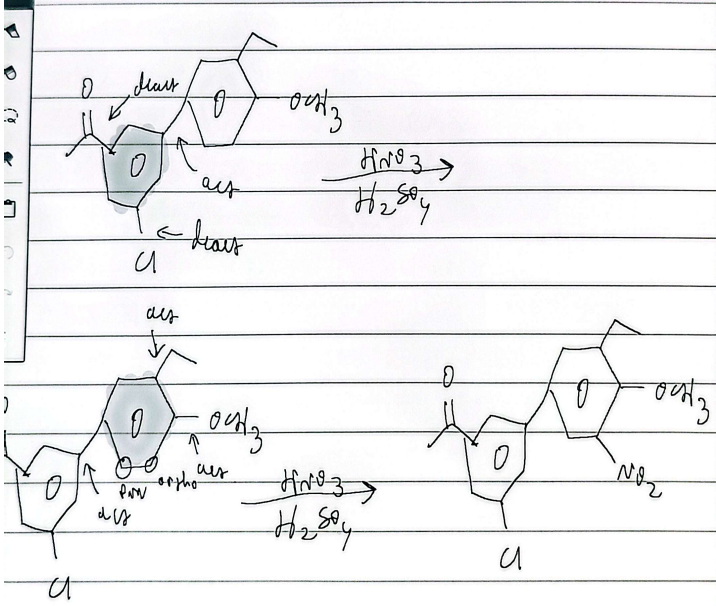
ring priority
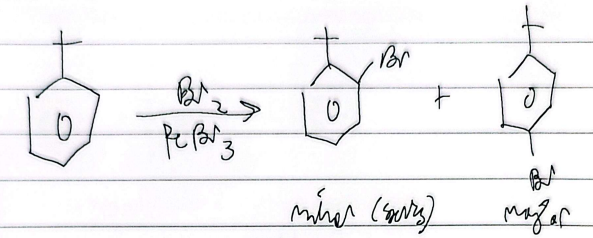
bromination
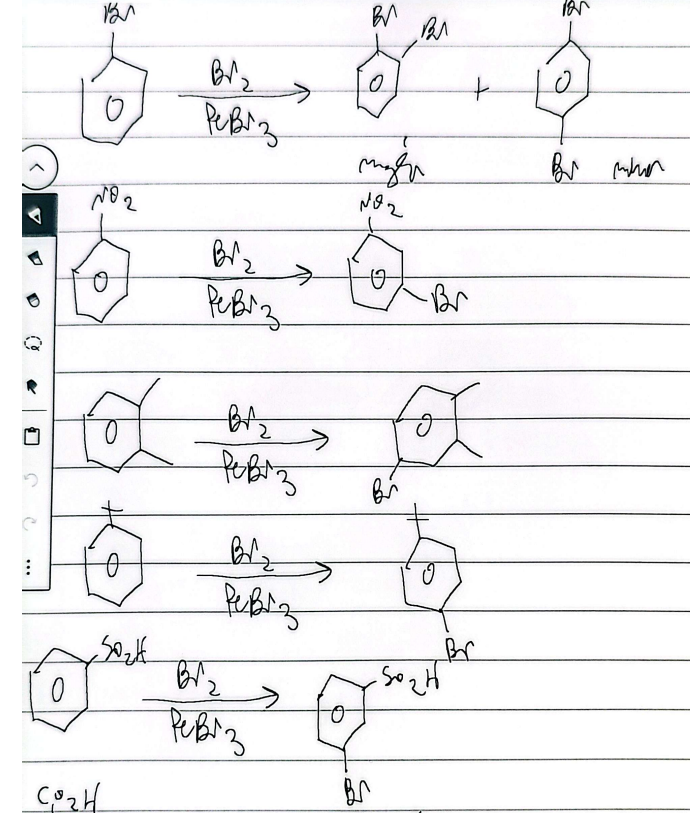
bromination
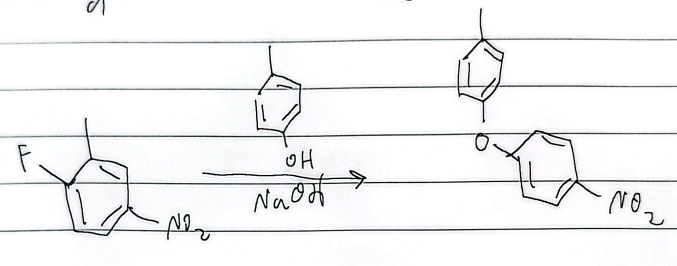
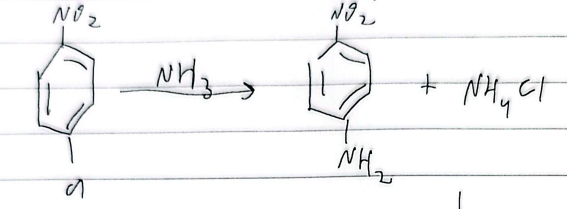
NAS (nucleophilic aromatic substitution)
NAS