Alcohols
1/7
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
8 Terms
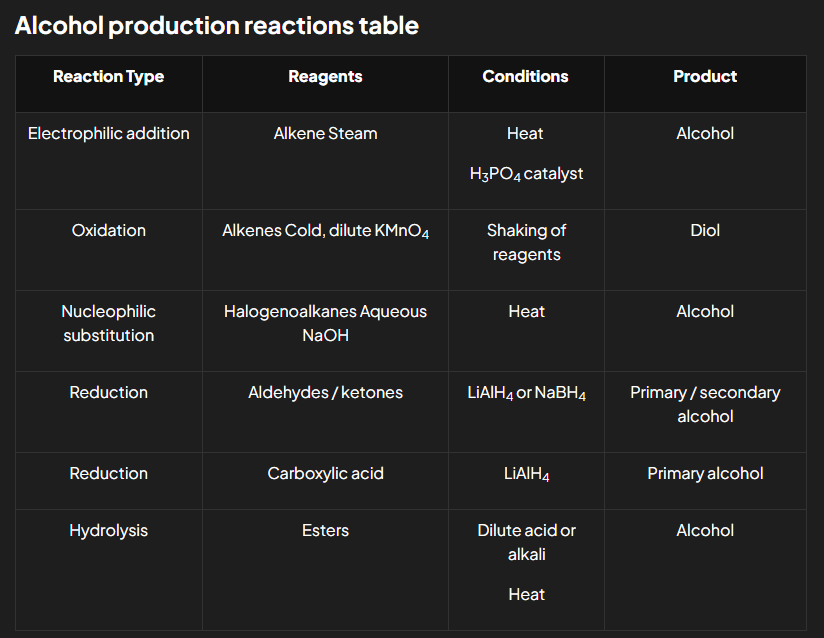
Production
Electrophilic addition of alkenes
Oxidation of alkenes
Nucleophilic substitution of halogenoalkanes
Reduction of carboxylic acids
Hydrolysis of ester
Substitution of alcohols
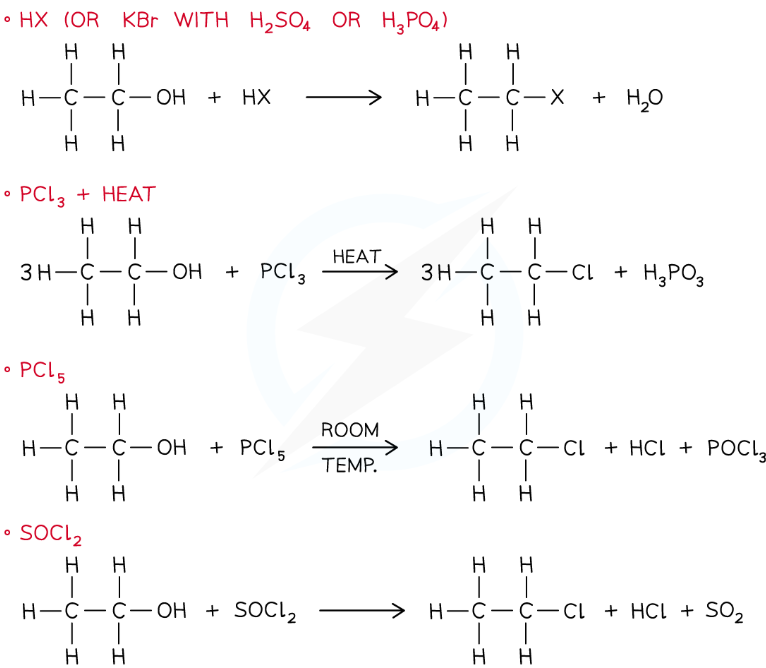
In the substitution of alcohols, a hydroxy group (-OH) is replaced by a halogen to form an halogenoalkane
The substitution of the alcohol group for a halogen can be achieved by reacting the alcohol with:
HX (rather than using HBr, KBr is reacted with H2SO4 or H3PO4 to make HBr that will then react with the alcohol)
PCl3 and heat
PCl5 at room temperature
SOCl2
Reaction with Na
When an alcohol reacts with a reactive metal such as sodium (Na), the oxygen-hydrogen bond in the hydroxy group breaks
Though the reaction is less vigorous than sodium reacting with water, hydrogen gas is given off and a basic compound (alkoxide) is formed
If the excess ethanol is evaporated off after the reaction a white crystalline solid of sodium alkoxide is left
Alcohol + sodium → sodium alkoxide + hydrogen
Oxidation of alcohols
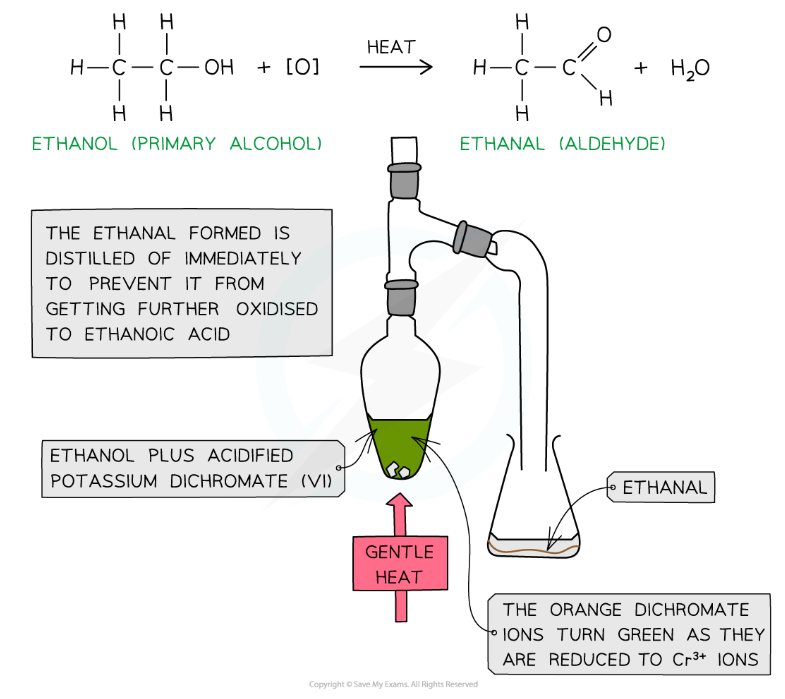
Primary alcohols can be oxidised:
They form aldehydes which can undergo further oxidation to form carboxylic acids
Secondary alcohols can be oxidised
The form ketones only
Tertiary alcohols do not undergo oxidation
Oxidising agents
The oxidising agents of alcohols include acidified K2Cr2O7 or acidified KMnO4
This reduction requires hydrogen (H+) ions which are provided by the acidic medium
When alcohols are oxidised the orange dichromate ions (Cr2O72-) are reduced to green Cr3+ ions
Products
When a warm primary alcohol is treated with an oxidising agent, it is initially oxidised to an aldehyde
The aldehyde has a lower boiling point than the alcohol, so it can be distilled off as it forms to prevent further oxidation
If it is not distilled, continued heating under reflux with excess oxidising agent will convert the aldehyde into a carboxylic acid
In contrast, secondary alcohols are oxidised to ketones, which cannot be further oxidised, so there is no need to distil them off immediately
Dehydration of alcohols
Alcohols can also undergo dehydration to form alkenes
Dehydration is a reaction in which a water molecule is removed from a larger molecule
A dehydration reaction is a type of elimination reaction
Test
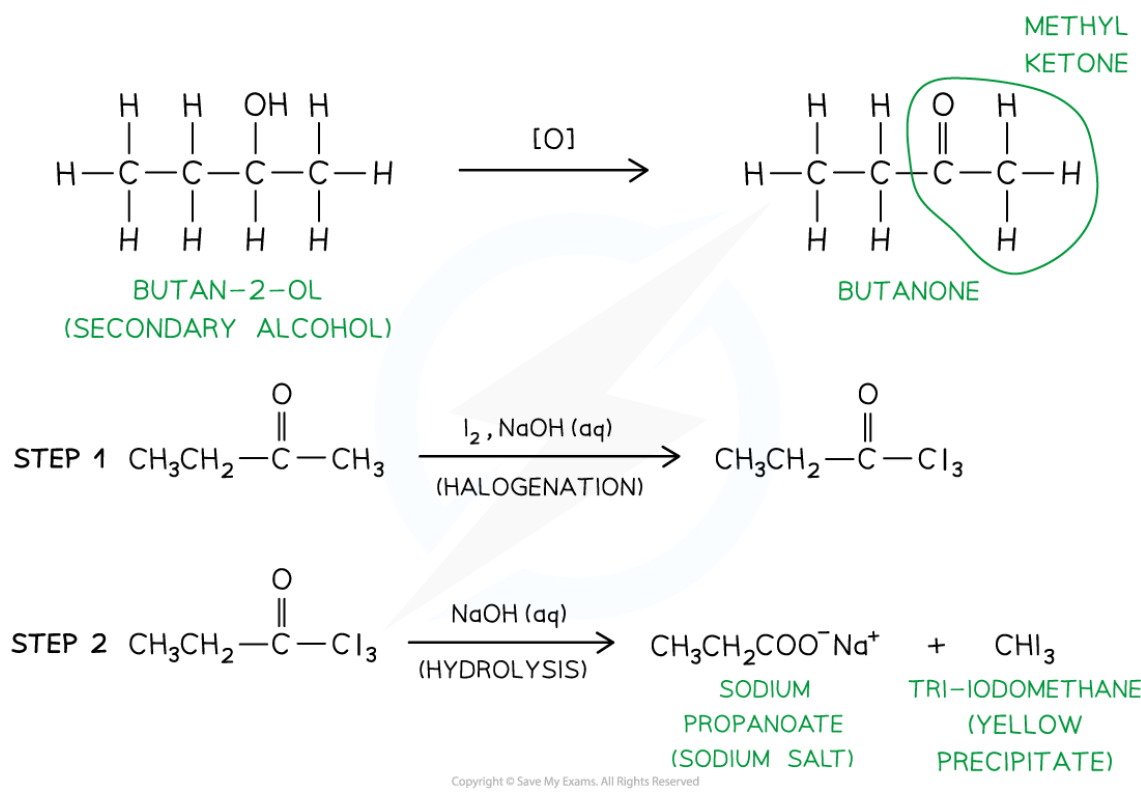
Tri-iodomethane (also called iodoform) is a yellow precipitate
The position of a secondary alcohol group in a molecule can be identified using the iodoform test with iodine in alkaline solution
If the –OH group is attached to a carbon atom that is next to a methyl group, the alcohol can be oxidised by the alkaline iodine to form a methyl ketone (RCOCH3)
The methyl ketone then undergoes:
Halogenation, where the three hydrogen atoms of the methyl group are replaced by iodine atoms (–CI3), followed by
Hydrolysis, forming a sodium carboxylate salt and a yellow precipitate of iodoform (CHI3)
A positive result (yellow precipitate) indicates that the alcohol has the structure CH₃CH(OH)R
No precipitate means the secondary alcohol is not adjacent to a methyl group and therefore cannot form a methyl ketone intermediate