5D- Lattice enthalpy and free energy
1/45
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
46 Terms
define lattice enthalpy
the energy change that accompanies the formation of one mole of ionic compounds from gaseous ions under standard conditions
are lattice enthalpies exothermic or endothermic
exothermic (negative) as they involve bond formation
define enthalpy change of formation
the enthalpy change when one mole of a compound is formed from its elements under standard conditions, with all reactants and products in their standard states
define enthalpy change of atomisation
the enthalpy change that takes place when one mole of gaseous atoms forms from the element in its standard states
is enthalpy change of atomisation exothermic or endothermic
endothermic (positive) as bonds are being broken
define first ionisation energy
the energy required to remove one electron from each atom in one mole of gaseous atoms to form one mole of gaseous 1+ ions
define first electron affinity
the enthalpy change that takes place when one electron is added to each atom in one mole of gaseous atoms to form one mole of gaseous 1- ions
is first electron affinity exothermic or endothermic
exothermic (negative) because the electron being added is attracted to the positive nucleus. the nuclear attraction is stronger than the electron repulsion
are second/third electron affinities exothermic or endothermic
endothermic (positive) the electron is being added to a negative ion so is repelled. energy is required to overcome this repulsion
how can lattice enthalpy strengths be compared
based on strength of ionic bond. stronger attractions in lattices with smaller ions and higher charged ions
what do Born-Haber cycles look like
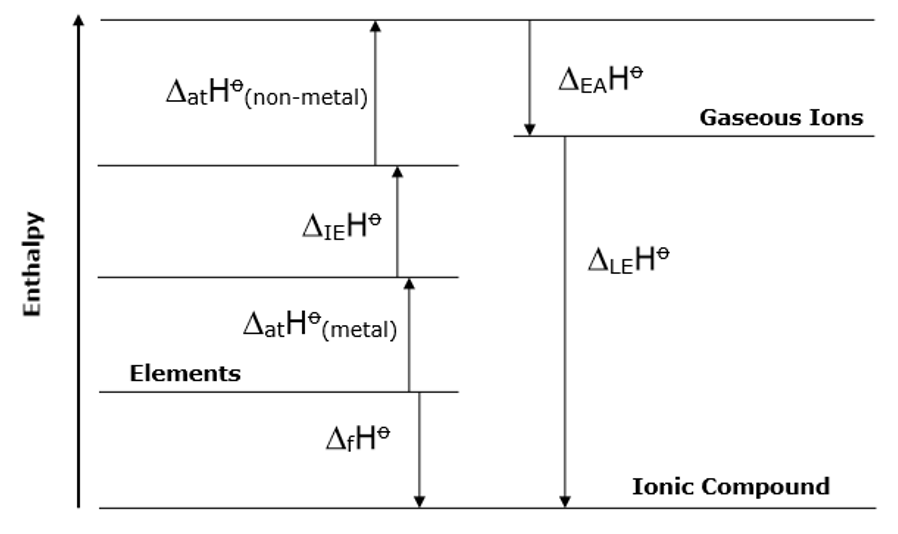
what does Hess’s Law state
if a reaction can take place through more than one route and the initial and final conditions are the same, the total enthalpy change is the same for each route
in a Born-Haber cycle for enthalpy of solution what should be at the top
gases as they have the most energy
define standard enthalpy change of solution
the enthalpy change that takes place when one mole of a compound is completely dissolved in water under standard conditions
define standard enthalpy change of hydration
the enthalpy change that takes place when one mole of isolated gaseous ions is dissolved in water forming one mole of aqueous ions under standard conditions
is standard enthalpy change of hydration exothermic or endothermic
exothermic as new electrostatic attractions are being made on hydration
is standard enthalpy change of solution exothermic or endothermic
either
what happens when a solid dissolves
the ionic lattice breaks down into gaseous ions, the ions become hydrated
what does the value of enthalpy change of solution depend on
the balance between the ionic lattice breaking down into gaseous ions and the ions becoming hydrated
what factors affect lattice enthalpy
ionic charge and ionic radius
how will lattice enthalpy change as radius of a cation decreases
it will become more exothermic as the charge will be distributed over a smaller volume so the attraction for the negative ion will increase
how will lattice enthalpy change as radius of an anion increases
lattice enthalpy will be less exothermic as the charge is distributed over a larger volume so the attraction for the positive ions will decrease
what factors affect enthalpy change of hydration
size of ions and charge of ions
what type of ions have the most exothermic lattice enthalpy/enthalpy of hydration
small ions with high charges
what is the process of hydration
forming bonds with water molecules, the stronger these bonds the more negative the enthalpy of hydration would be
why do ionic charge and ionic radius affect the magnitude of lattice enthalpy and enthalpy of hydration
they both affect charge density- the amount of electric charge per volume, ions with small radii and high charges will have the greatest charge density
what is the relationship between the charge on the ions, the size of the ion and the charge density
charge density= ionic charge/ionic volume
how does lattice enthalpy change as the charge density on an ion decreases
lattice enthalpy becomes more negative
define entropy
a measure of the dispersal of energy is a system, the more disordered a system the greater the entropy
what is the symbol for entropy
S
what are the units for entropy
J K^-1 mol^-1
is entropy positive or negative
positive
how do you calculate entropy change
sum of entropy of products - sum of entropy of reactants

are changes in entropy positive or negative
either
are positive or negative changes in entropy favoured
positive- processes which involve an increase in disorder
What does Gibbs free energy show
ΔG, used to quantitatively decide if a reaction is feasible
what is a feasible reaction
one that occurs without a continuous supply of energy
what value of ΔG are processes feasible
if ΔG is negative
what is the equation for Gibbs free energy
ΔG = ΔH - (T x ΔS)
ΔG- Gibbs free energy (kJ mol^-1)
ΔH- enthalpy change (kJ mol^-1)
T- temperature (K)
ΔS- entropy change (kJ K^-1 mole^-1)
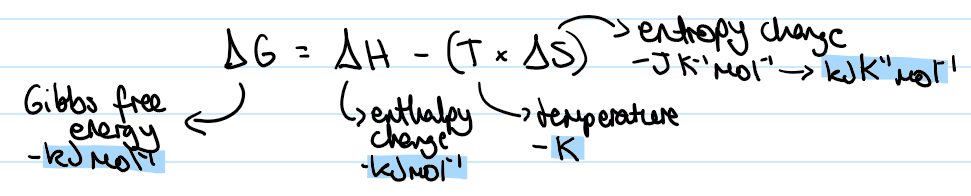
why might a feasible reaction not appear to occur
rate of reaction is too slow due to a high activation energy
what is there is a process requires a high or low temperature in order to be feasible
there will be a minimum or maximum temperature at which the process is feasible, the change occurs at the point that ΔG=0
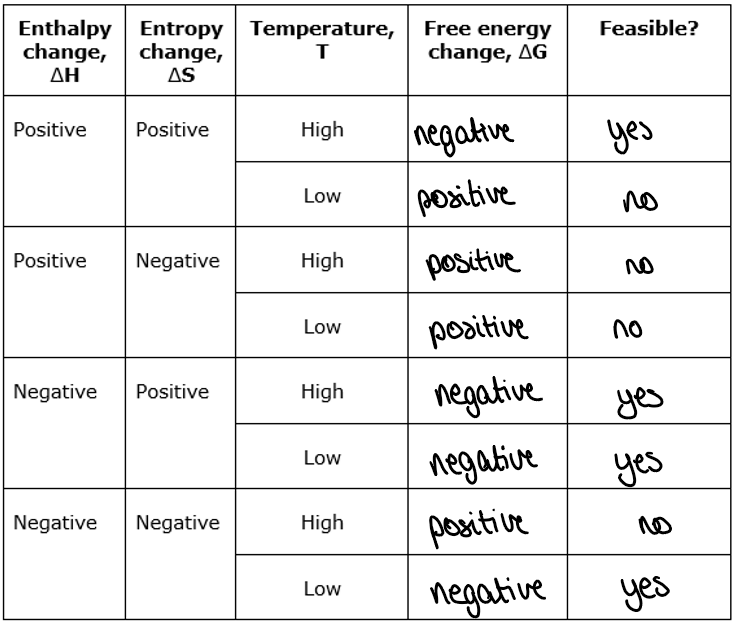
enthalpy change and entropy change signs and temperature and whether reaction is feasible
how the Gibbs free energy equation be rearranged for graphical use
ΔG = -ΔS x T + ΔH
y = m x + c
in a graph of Gibbs free energy against temperature what does the y-intercept indicate
ΔH (enthalpy change)
in a graph of Gibbs free energy against temperature what does the x-intercept indicate
the temperature when the reaction become feasible
in a graph of Gibbs free energy against temperature what does the gradient represent
-ΔS (negative entropy change)