Instrumental Analysis Exam 3 CHEM 3334
1/106
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
107 Terms
Peak Generation
Repetitive distribution depends on the relative phase movement
Peak Broadening
Nonequilibrium distribution depends on the relative phase movement
Peak Broadening can be induced by molecular diffusion
Can be expressed in terms of flux:
Flux (mol/m² * s) —> J=-DM (dC/dx)
DM= molecular diffusion coefficient
dC/dx = concentration gradient
x= distance
If diffusion exhibits normal/random distribution then:
C(mol/m3)= (mol/sqrt(4piDMt)) * e-x2/(4Dmt)
Plate Theory of Chromatography
sigma2 /x = 2Dm /ux = H
Plate Theory Definitions
H is the plate height (or height equivalent to a theoretical plate) hetp
Variable N
The number of theoretical plates is also a measure of column efficiency: the greater the number, the narrower the bandwidth, the better the resolution.
Equation for number of theoretical plates:
Nt=L/H
L= is the column length
H= is the plate height
The longer the column, the greater N
The smaller the plate height, the greater N
Substituting H= sigm2/x
Nt= L * x/sigma2=L² /sigma²
Considering a certain analyte that provided a peak with a given tr
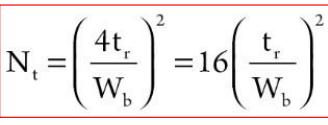
Wh (full width at the half maximum WFWHH) can be measured more accurately than Wb
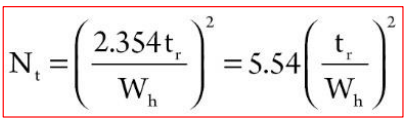
Q. How can we decide on the number of theoretical plates? How can we decide how many plates we need to get a good separation?
It is crucial to be able to estimate the resolution necessary to solve the problem, extent of separation between two adjacent solutes
Resultion
Extent of separation between two adjacent bands/peaks/solutes
Resolution Equaiton
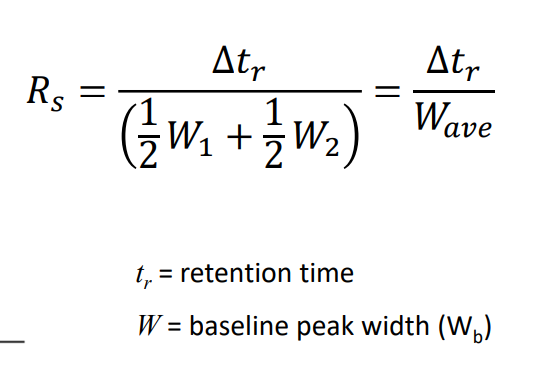
Resolution when there is only one peak
Rs = tr/W50%
W50% can be the width of the peak at 50% height, the full width at half height, or the full width at half maximum (all the same damn thing)
Resolution is proportional to the square root of the number of theoretical plates!

Rate theory of chromatography
band spread is the result of cumulative random processes
variance is additive
Processes can be divided into 2 categories
on column (processes occur during separation)
off-column (pre/post column) either at injection port or post detector
Rate theory of Chromatography central equation
HT=HE+HD+HS
E= eddy diffusion/multiple flow paths
D=Longitudinal (molecular) diffusion
S= equilibration between phases (mass transfer)
Eddy Diffusion
Relates to the different paths that analyte might take through the stationary phase support particles
Eddy diffusion caveats
assumes that the column is not effected by mobile phase flow rate
more possible paths lead to greater peak broadening
minimized by small, uniformly packed SP particles
However, smaller particles leads to tighter packing, so greater pressures are needed to push the mobile phase through column
Longitudinal Diffusion (thermodynamic and kinetic)
sigma2=B/ux
B=constant f (column stationary phase, etc_
ux= flow rate
Longitudinal Diffusion
this is a natural diffusion from a region of high concentration (center of band) to a low concentration (beyond the band). It occurs regardless of flow rate. A higher flow rate means less time in column, hence a lower band broadening
Mass transfer (kinetic)
There occurs equilibration between phases: the solute in the stationary phase lags behind the rest of the solute zone as it travels down the column.
Competition of mass transferring between phases and mass transport along with mobile phase. High flow rate causes analyte molecules in th eMP to move away from those partitioned into SP, increasing broadening.
Mass transfer (kinetic) equation
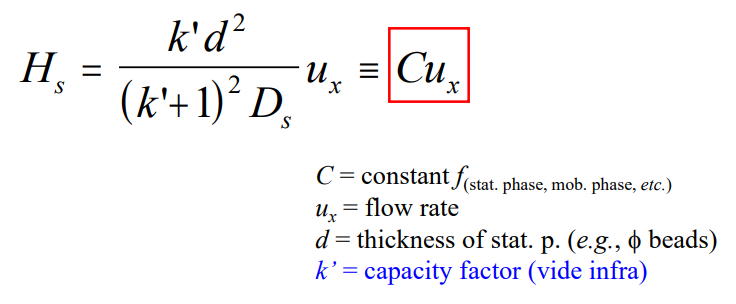
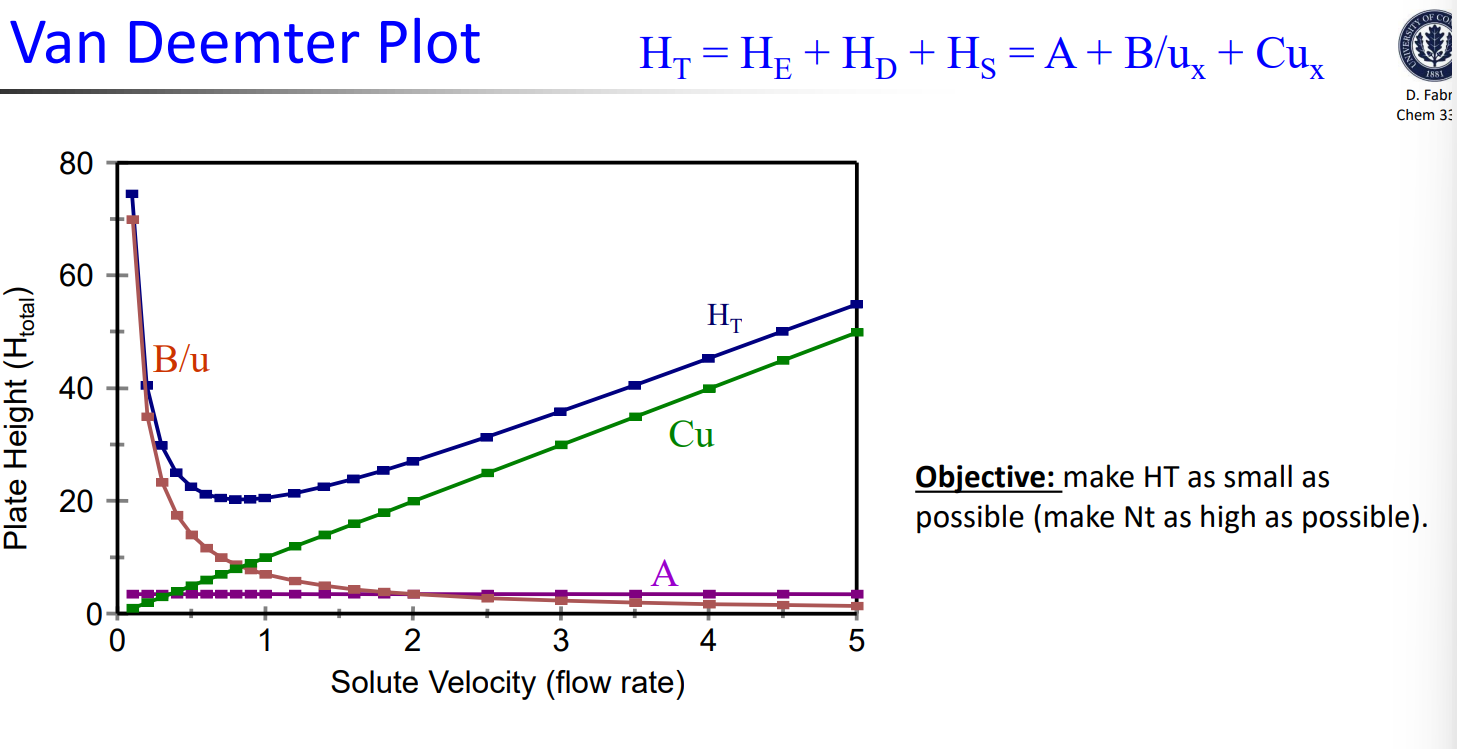
Van Deetmer Equation****
HT=A+B/ux+Cux
A=eddy diffusion
B/ux= longitudinal (molecular) diffusion
Cux= equilibration between phases (mass transfer)
Van Deetmer Plot example:
Retention time
Time needed after injection for component to elute
Adjusted retention time
tr’=tr=tm
tm=void/dead volume
Relative retention
alpha=tr’ (2)/tr’ (1)=K2/K1
K1 and K2 are partition coefficients for solute 1 and 2
Partition coefficient
K=Cs/Cm
Cs is the concentration of solute in stationary phase
Cm is the concentration of solute in mobile phase
Retention (capacity) factor (k’)
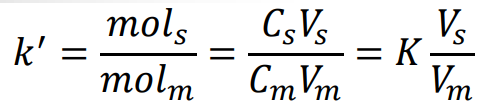
Alternative Retention (capacity) factor (k’)
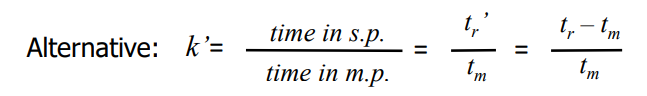
Retention Volume
volume of mobile phase needed to elute solute
Vr=tr*Fmp=Vm + K*Vs
Vr’=Vr-VmK*Vs
Fmp=flow rate
Vm=mobile phase volume
Vs=stationary phase volume

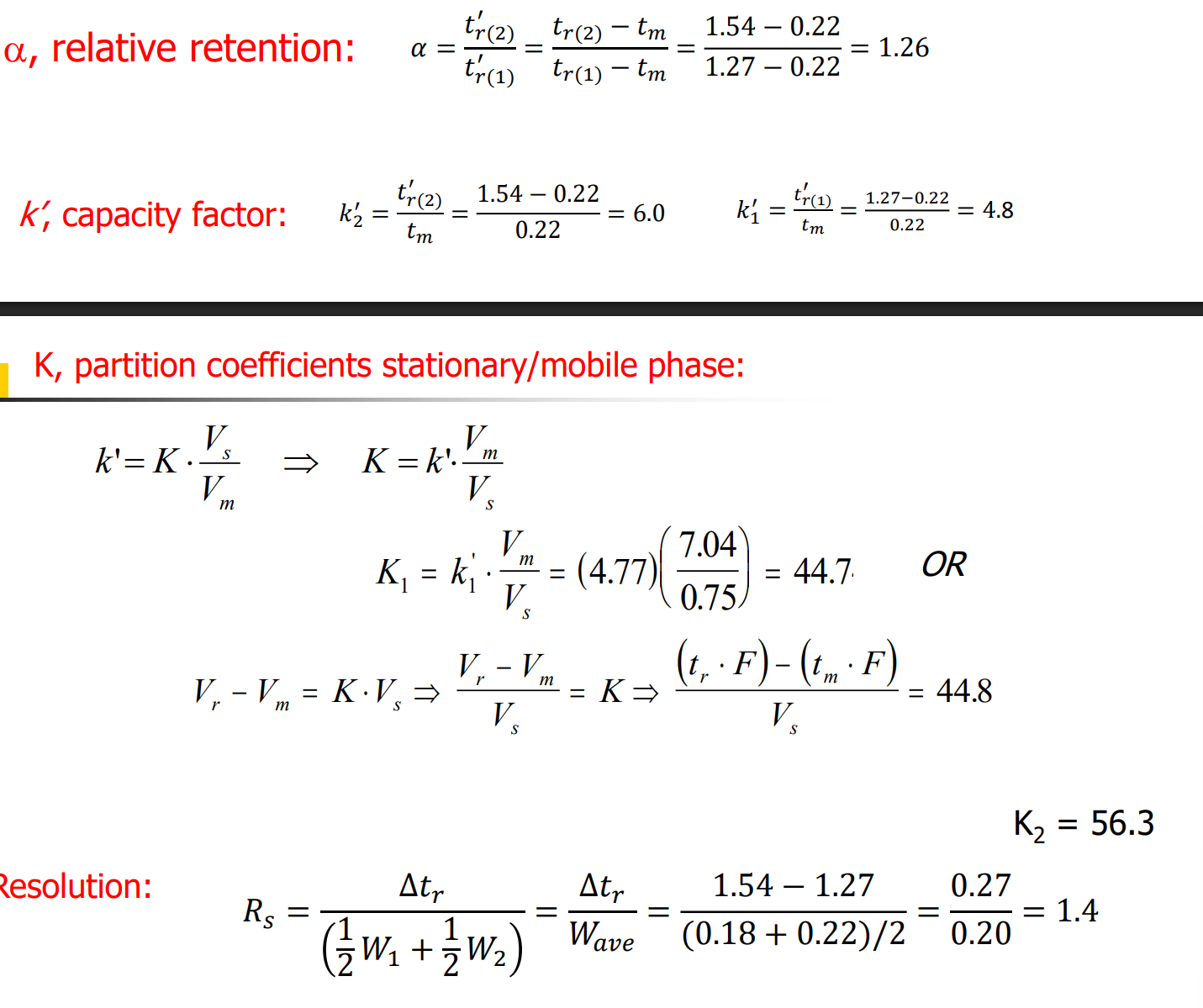
Exam problem 1:
answer:
Different types of chromatography
Absorption chromatography
partition chromatography
ion exchange chromatography
Size exclusion
Affinity Chromatography
Absorption chromatograhpy
Solid stationary phase and a liquid or gas mobile phase
solutes absorb onto surface of stationary phase (GSC, LSC, TLC)
Partition Chromatography
Liquid/polymeric stationary phase and a gas/liquid mobile phase. Solutes partition onto stationary phase (GC, GLC, HPLC)
Ion exchange chromatography
Stationary phase is ion exchange resin; liquid (aqueous) mobile phase. Separation based on electrostatic (charge) interactions
Size exclusion chromatography
stationary phase is porous gel; liquid mobile phase. Separation is based on size so, larger molecules are excluded from pores of gel, travel faster through system.
SHAPE IS MORE IMPORTANT THAN SIZE/MW
Affinity Chromatography
used predominantly for separation/isolation of biochemicals/proteins. There is a liquid mobile phase. The stationary phase consists of specific proteins/compounds covalently linked to solid phase. (for example antigen/antibody; enzyme/substrate-cofactor)
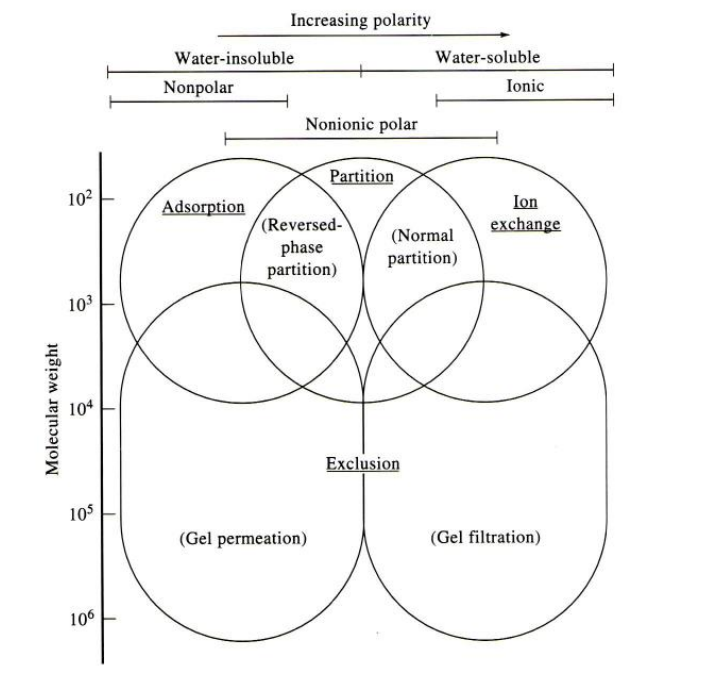
Chromatography selection chart
separation based on physical and/or chemical features such as size and polarity.
Chromatography workflow:
choose stationary phase (theoretical plates/resolution)
choose a mobile phase (theoretical plates/resolution)
decide how much to inject (capacity factor)
dial a flow rate (van deetmer equation)
run
evaluate results
adjust conditions
repeat
Gas Chromatography Increasing resolution
increase N by increasing the partitioning surface
Gas Chromatography (increasing selectivity)
increase deltaK by adjusting the chemistry
GC detectors
Flame ionization detector
thermal conductivity detector
differential amplifier for TCD (twin sample and reference cells)
Electron capture detector
Flame ionization detector (FID)
detects organic compounds by burning the effluent in a hydrogen air flame producing ions. the resulting current is electrodes is proportional to the number of carbon atoms. Highly sensitive to hydrocarbons but insensitive to water and permanent gases.
Thermal Conductivity detector
measures changes in thermal conductivity of the carrier gas by teh presence of analyte molecules. it is universal and non-destructive, but less sensitive than other detectors.
Differential amplifier for TCD
Enhances TCD sensitivity and stability by comparing the thermal conductivity of the column effluent (sample cell) to that of pure carrier gas (reference cell), using a differential amplifier to detect imbalances.
Electron Capture Detector
Detects electronegative compounds (like halogens) by measuring the decrease in current caused by analyte molecules capturing electrons from a radioactive beta-emitter. Very sensitive and selective to halogen compounds.
Atomic emission detector (AED)
uses a microwave induced plasma to excite atoms from eluted components. Provides both quantitative and qualitative information and can simultaneously detect multiple elements with high sensitivity
Supercritical fluid chromatography (SFC)
mobile phase is a supercritical fluid, substance with temperature and pressure above the critical point. Can have a solid or liquid stationary phase. Chromatographic characteristics intermediate between GC and LC. A supercritical fluid can dissolve samples soluble in liquids (not really volatile)
Reverse Phase Chromatography
Uses:
hydrophobic stationary phases
polar to non-polar gradients
As the gradient goes from polar to non-polar, hydrophobicity rules the order of elution
Size Exclusion chromatography (SEC)
Ve=Vo+KavVg
in order of variables, elution volume, void volume occupied by mobile phase, partition coefficient between gel and surrounding mobile phase, gel volume
Vt= total volume (void+gel)
Radius of gyration Rgyr=k(MW)a
a=0.1 for rods, 0.5 for flexible coils, 0.3 for spheres
GC vs LC
GC, mobile phase: carrier gas; Stationary phase solid or liquid. key parameters: temperature and polarity. limited choice of carrier gases, adjust temperature and flow rate only. perform a temperature gradient.
LC, mobile phase: liquid; Stationary phase: solid or liquid. key parameters: polarity and reactivity. broader choice of separation solvents/systems. Adjust pressure, flow rate and temperature. Perform gradients of organic content, salt pH, etc…
Both (stationary phase): packed and capillary columns used to increase SA. the longer the column the greater the number of theoretical plates.
Spectroscopy
studies the interaction between electromagnetic radiation, energy, and matter
Wave Properties:
characterized by v, lambda, nu, I = A sin (pi nu t + phi), E = h nu, P, etc
additive nature, destructive/constructive
diffraction when crossing a slit or pinhole
refraction and reflection when crossing boundary between media
transmission absorption, scattering, polarization when crossing a specific medium
Particle properties
The photoelectric effect
Wavelength equation
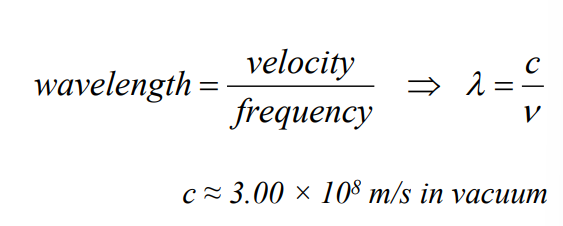
coherent radiation
the phases of two or more waves representing the radiation differ by a known constant that does not change over time (RF oscillators, microwave sources, optic lasers)
Incoherent radiation
the phase differences between two or more waves are unknown or random (tungsten bulb)
Radiation refraction
radiation passes at an angle through interface between two transparent media
Radiation reflection
when radiation crosses interface between media with different n. The reflected fraction increases with delta n. for a right angle beam:
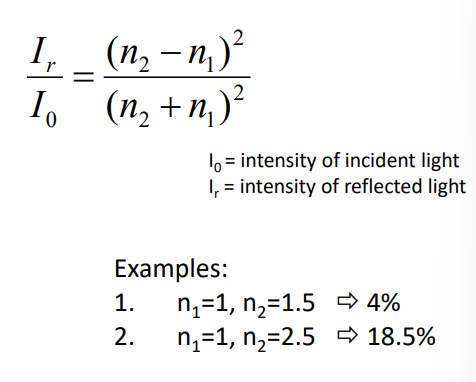
Radiation scattering
due to re-emission of light after interaction. Absolute destruction for small molecules, partial interference for larger ones.
Rayleigh Scattering
molecules «lambda —> which is the reason why the sky is blue due to greater scattering of short wavelengths
Large molecule scattering
used to determine size and shape of molecules
Raman Scattering
Used to determine size and shape of molecules
Radiation Polarization
removal of all but one of the possible vibrational planes
atomic absorption
atoms in gas phase
only electronic
Molecular Absorption
molecules
electronic, vibrational and rotational data
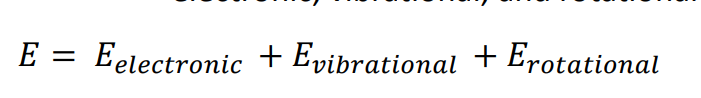
Non-radiative relaxation
energy loss in small steps, collisions
Radiative relaxation
fluorescence relaxation: t < 10-5 s
phosphorescence relaxation: t > 10-5 s
Emission
Emitted Pe
Pe=kc
atomic emission
Luminescence
P1
P1=kc
Atomic and molecular fluorescence, phosphorescence, and chemiluminescence
Scattering
Psc=kc
Raman scattering, turbidimetry, and particle sizing
Absorption
Incident, P0 and transmitted, P
-logP/P0=kc
Absorption process
determines the part of the incident power that is transmitted (not absorbed as a function of frequency/wavelength
Emission happens in two steps
excitation: stimulus energy is applied in the form of light, heat, electrical energy, particles, chemical reaction
Emission: electromagnetic radiation is returned
Chemoluminescence
excitation by thermal, electrical or chemical stimulus (non-radiative)
Photoluminescence
excitation by radiation: fluorescence and phosphorescence
Fluorescence
prompt re-emission
Phosphorescence
delayed re-emission
Elastic Scattering
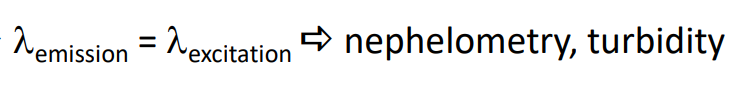
Inelastic scattering
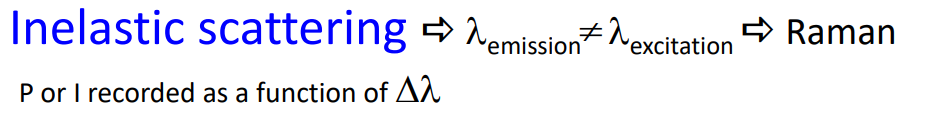
Emission Spectra
when excited electrons relax back, their excess energy is released as photons
Bands: electronic and vibrational levels (small molecules)
Lines: only electronic (atoms, ions)
Discrete lines
indicate an atom
unresolved lines
indicate a molecule
Continuum
is produced when solids are heated up: blackbody radiation
Blackbody Spectrum
BB radiation is emitted when a solid is heated to incandescence
produced by thermal excitation and relaxation of many vibrational (and rotational) levels
temperature dependent
different materials exhibit different ranges
Can be used as a light source with very broad emission ranges
Blackbody absorption happens when
0% of radiation is transmitted or reflected and 100% is absorbed
Absorption quantitative aspect
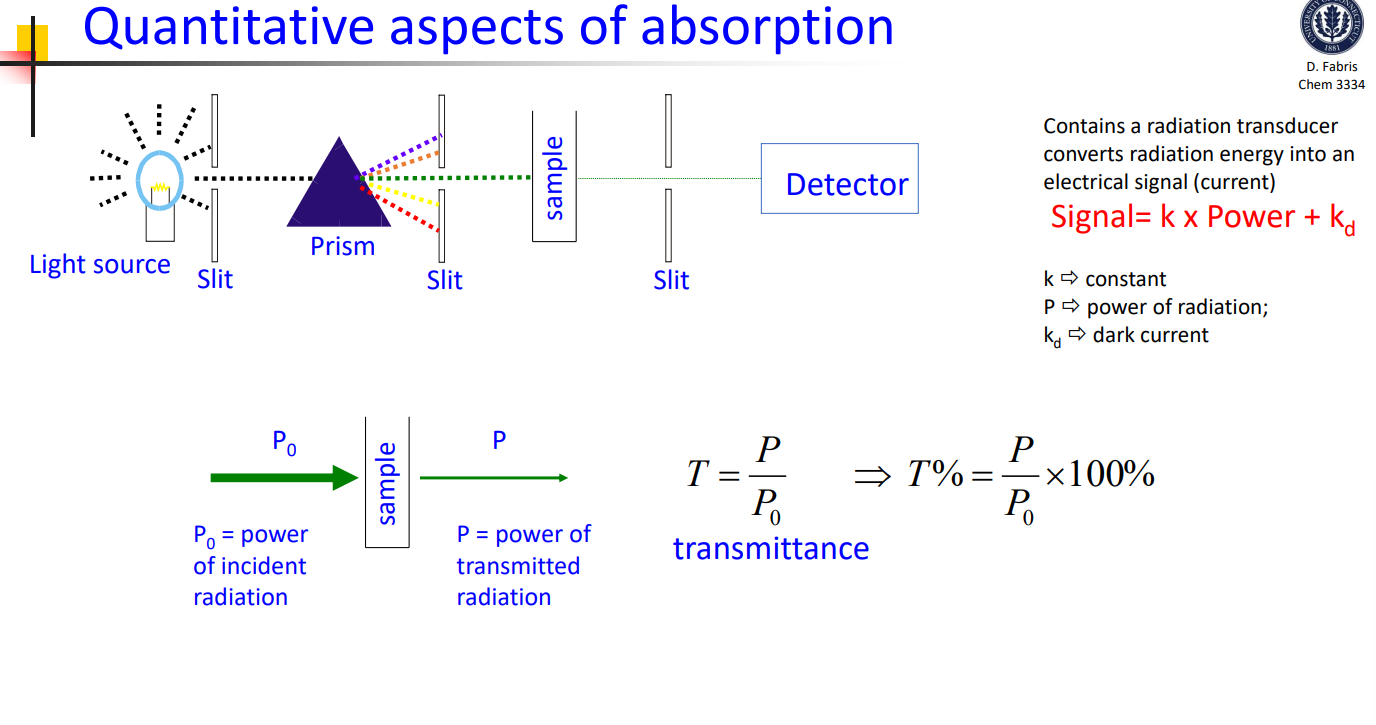
Transmittance
P/P0
Absorbance
=logP0/P=log1/T=-logT
No light absorbed
P=P0
T=1
A=0
All light absorbed
P=0
T=0
A= infinity
90% of light absorbed
P=10%
T=10%/100%=0.1
A=-log(0.1)=1
Lambert’s law
T=P/P0=10-kb
Beer’s Law
T=P/P0=10-k’c
Beer-Lambert’s Law
-log T =log P0/P=abc=A
B-L law in terms of transmittance
A=2-logT%
Scatchard plot for the det of equilibrium constants