Chapter 2 – Chemistry of Life: Part 1, Inorganic Chemistry
1/32
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
33 Terms
Chemical Element
composed of only one type of atom (e.g., hydrogen, nitrogen, sulfur, aluminum)
Elements are arranged in a periodic table
Atom
the smallest units of an element that maintain the properties of the element
Protons
Have a positive charge; located in the nucleus
Neutrons
Have no charge; located in the nucleus
Electron
Have a negative charge; located in electron shells .
When is an atom Neutral?
An atom has the same number of protons and electrons, An atom is neutral because the positive and negative charges cancel each other.
Atomic Symbol
a shorthand method for representing an element, e.g., C, Mg, Na, Often derived from the Latin name of the element (e.g., Na = natrium, which means sodium in Latin)
Atomic Mass
is the mass of an atom determined by the number of protons and neutrons .
· Protons and neutrons both have an atomic mass of 1 atomic mass unit. Electrons have negligible mass, which is why electrons do not contribute to atomic mass of an atom.
· The atomic mass is not a whole number because it is the average mass of all isotopes of an element.
Atomic Number
is the number of protons an atom contains
Isotopes
are atoms of the same element that have a different number of neutrons, The atomic mass of an element is the average mass of all isotopes of an element.
Hydrophillic
Molecules that can attract water can dissolve in water these molecules are those that have a difference in charge distribution within them (i.e., regions of positive and regions of negative), This includes ionic molecules (salts) and molecules with polar covalent bonds (e.g., sugars), The positive regions can attract the slightly negative oxygen atoms within water, and the negative regions can attract the slightly positive hydrogen atoms within water.
Hydrophobic
molecules that cannot attract water can dissolve, these molecules are those that do not have regions of positive and negative charge within the molecule (that is, they have an equal charge distribution), This is molecules that contain mostly non-polar covalent bonds (e.g., oils and fats
Radioistopes
unstable isotopes, They change to a stable form by emitting radiation (which is energy in the form of waves or particles), Radioactivity is the ability to emit radiation, Radioisotopes have important uses (e.g., medical imaging, food sterilization, cancer therapies), but a high level of radiation is dangerous, High energy radiation can damage our genetic material (DNA) and other molecules inside cells.
pH
very important for the proper functioning of cells and organisms, All cellular proteins function within a very narrow pH range, All chemical reactions occur within a very narrow pH range, e.g., the pH of normal blood is 7.4. It can be fatal quickly if the pH gets much lower or much higher than 7.4.
Most of the mass of an organism (including humans) is composed of what 4 elements?
hydrogen, oxygen, nitrogen, and carbon
Mineral and Trace Elements
There are other elements that are needed in small quantities for healthy function and are called mineral elements (7 elements) and trace elements (14 elements). E.g., calcium and phosphorus are needed for healthy bones, E.g., sodium and potassium and needed for neurons to transmit nerve impulses, E.g., iron is needed for red blood cells
Acids
An acid is a molecule that increases H+ (hydrogen ion) concentration, e.g., hydrochloric acid, HCl. It breaks apart to release H+ and Cl- ions and therefore increases the concentration of H+ ions, Acidic solutions have a higher H+ (hydrogen ion) concentration compared to water, Acidic solutions have a pH less than 7
Bases
An base is a molecule that decreases H+ (hydrogen ion) concentration, e.g., sodium hydroxide, NaOH. It breaks apart to release OH- and Na+ ions. The released OH- ions then remove H+ ions from solution, (to form water molecules), therefore decreasing the H+ (hydrogen ion) concentration Basic solutions have a lower H+ (hydrogen ion) concentration compared to water.
Basic solutions have a pH
Molecules
A molecule is 2 or more bonded together, Each molecule has a molecular formula, It contains chemical symbols for the elements in the molecule, It contains numbers to indicate the number of each type of atom, If a number is not present next to an atom, it means there is only 1 atom present, e.g., H2O = molecular formula for water (1 oxygen atom bonded to 2 hydrogen atoms)
Compound
a molecule composed of 2 or more elements., e.g., H2O is a compound composed of hydrogen and oxygen atoms, O2 is molecular oxygen composed of 2 oxygen atoms. It is not a compound.
Chemical Bonds
with an unfilled outer electron shell form chemical bonds with their valence electrons, In a chemical bond an atom shares, releases, or obtains electrons to obtain a full outermost electron shell., There are 2 types of chemical bonds: Ionic bonds and Covalent bonds
Octet Rule
atoms are most stable when the outermost electron shell (called the valence shell) is full of electrons. For most atoms, the outermost shell is full when it contains 8 electrons For hydrogen and helium, the outermost shell is full when it contains 2 electrons.
Ionic bonds
In ion is an atom that has gained or lost electrons, One atom loses one or more electrons to satisfy the octet rule and produce a full outermost electron shell. This produces a postively charged ion because it now has more protons than electrons, Another atom gains the electrons to satisfy the octet rule and produce a full outermost electron shell. This leaves a negatively charged ion because it now has more electrons than protons, The two charged ions then bond together through the strong attractive force between the positive and negative charges, Ionic compounds are called salts, e.g., Common table salt is sodium chloride, NaCl
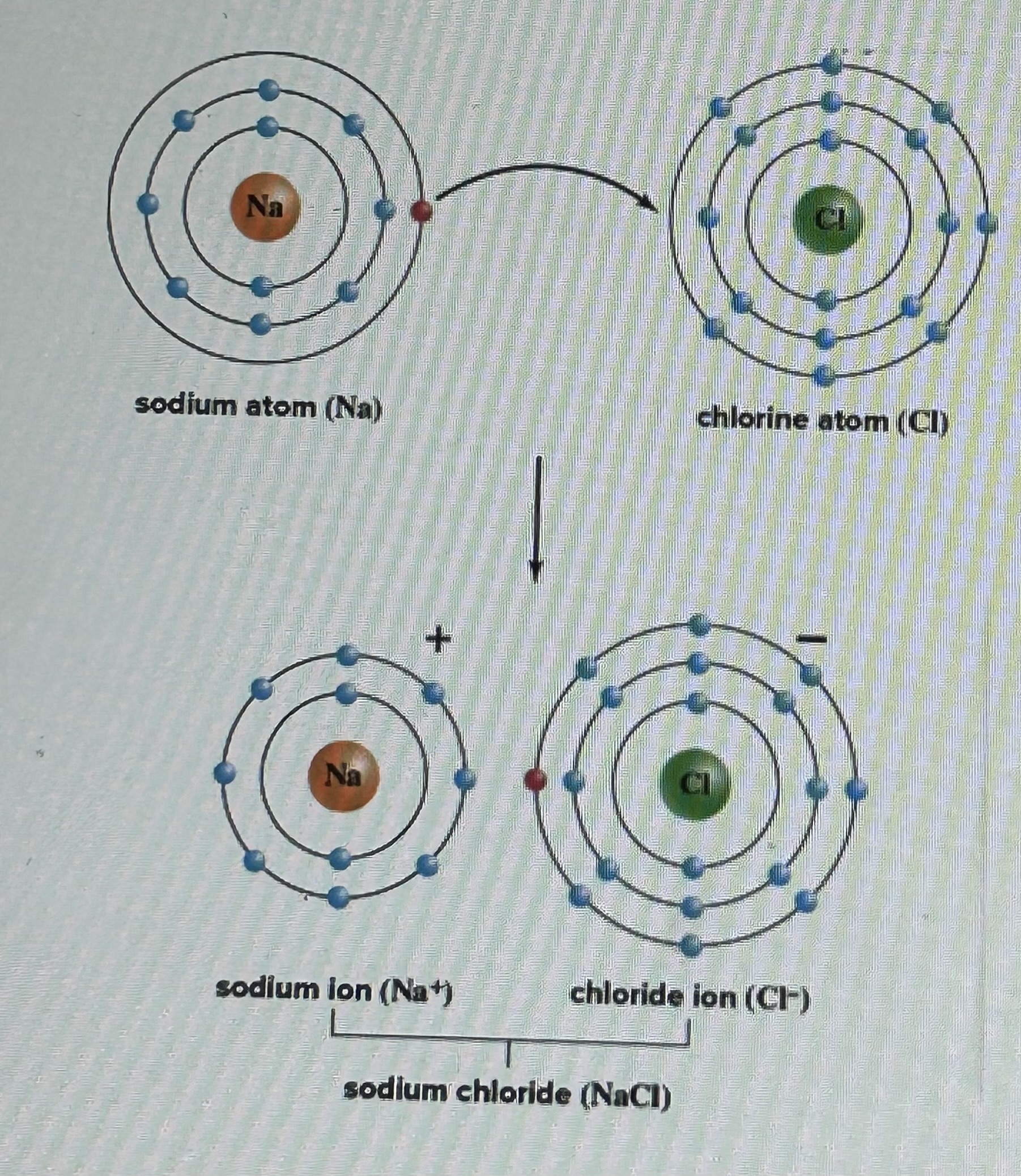
Ionic Bond Example
Sodium has 1 electron in the outermost shell, which does not satisfy the octet rule. It loses the electron. It now has 8 electrons in the outermost shell, which satisfies the octet rule. It is now a positively charged ion (because it has 11 protons and only 10 electrons).
Chlorine has 7 electrons in the outermost shell, which does not satisfy the octet rule. It gains the electron lost from sodium. Chlorine now has 8 electrons in the outermost shell, which satisfies the octet rule. It is now a negatively charged ion (because it has 17 protons and 18 electrons).The positively charged sodium ion and the negatively charged chloride ion associate together through the strong attractive force between positive and negative charges. This is an ionic bond.
Covalent Bonds
two atoms share electrons to achieve a full outermost electron shell, The shared electrons behave as if they belong to both atoms, The number of covalent bonds an atom will form is determined by how many electrons are needed to fill the outermost electron shell, e.g., oxygen needs 2 electrons to fill the outermost shell with 8 electrons. It will therefore form 2 covalent bonds with other atoms, 2 shared pairs of electrons, double bond, ex. O=O
Example- Water molecule H20- Covalent Bond
The oxygen atom has 6 electrons in the outermost electron shell. It needs 2 more electrons to satisfy the octet rule and have a full outer electron shell, Each hydrogen atom has 1 electron in the outermost electron shell. It needs 1 more electron to have a full outermost shell (recall, hydrogen and helium atoms have a full outer shell with 2 electrons), Therefore, the oxygen atom shares an electron with each hydrogen atom, and the hydrogen atoms each share their electron with oxygen, The result is that the oxygen atom forms a single covalent bond with each hydrogen atom (i.e., oxygen shares an electron pair with each hydrogen atom)
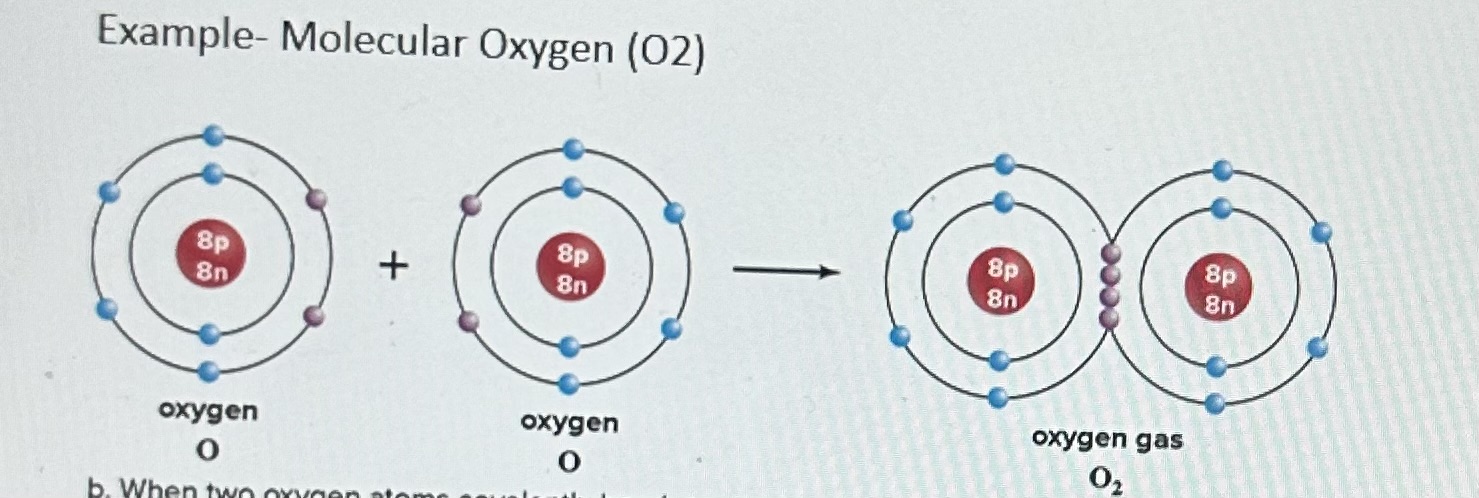
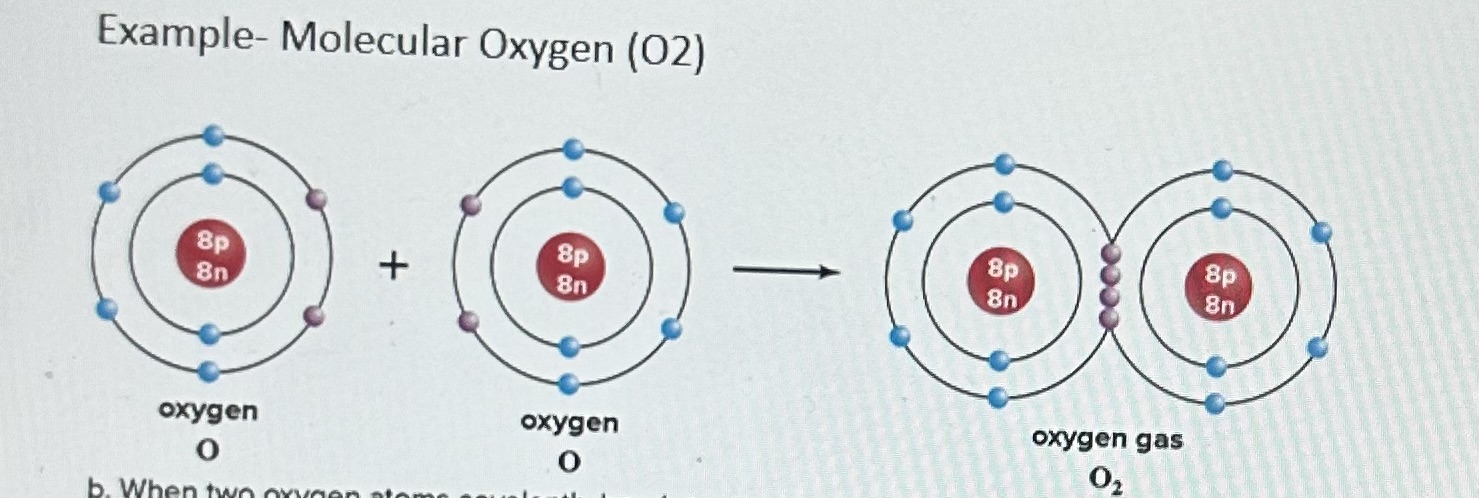
Example of Covalent Bonds- Molecular Oxygen 02
Each oxygen atom has 6 electrons in the outermost electron shell. It needs 2 more electrons to satisfy the octet rule and have a full outer electron shell, Therefore, each oxygen atom shares 2 electrons with the other oxygen atom, The result is a double covalent bond between the oxygen atoms (i.e., the oxygen atoms share 2 pairs of electrons with each other).
Polar Covalent Bond
In a polar covalent bond, there is unequal sharing or electrons between atoms. For example, the covalent bonds in water are polar covalent bonds. The oxygen atom has a stronger ‘pull’, or attraction, for the shared pair of electrons, so the electrons are closer to oxygen and further from hydrogen. Oxygen therefore gets a slight negative charge (depicted as δ-). The hydrogen atoms therefore get a slight positive charge (depicted as (δ+).
Polar Covalent Bond Example
Oxygen has a stronger ‘pull’, or attraction, for the shared pair of electrons, The shared electron pairs are therefore pulled closer to oxygen and farther from hydrogen,This creates an unequal charge distribution, or polarity, within the molecule, The oxygen atom has a slight negative charge, and each hydrogen atom has a slight positive charge.
Non-Polar Covalent Bond
there is equal sharing of electrons, Both atoms in the covalent bond have a similar ‘pull’ or attraction for the shared electrons, There is an equal charge distribution within the molecule.
Non-Polar Covalent Bond
Each oxygen atom has a similar pull for the shared electrons (yellow electrons in the middle), The electrons are therefore located equal distance from both atoms, There is an equal charge distribution, or polarity, within the molecule.
Hydrogen Bonds
A hydrogen bond is not actually a chemical bond, There is no loss or gain or sharing of electrons, A hydrogen bond is an attractive force between a slightly positive hydrogen atom from one molecule and a slightly negative atom from another molecule, e.g., hydrogen bonds form between water molecules, The slightly positive hydrogen atom of one water molecule is attracted to the slightly negative oxygen atom of a different water molecule.
Unequal Charge distribution
Electrons have not been lost or gained as in an ionic bond. The electrons are still shared, but the electrons are closer to one atom and farther from the other, which creates an unequal charge distribution referred to as ‘polarity’) within the molecule.