BIO TOPIC 2 TO REMEMBER
1/102
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
103 Terms
Main symptoms of CF
(digestive system)
Impaired digestion
Damage to pancreas
Mucus blocks pancreatic duct & small intestine
Digestive enzymes cannot reach duodenum → reduced digestion of food + absorption of nutrients
Trapped digestive enzymes damage pancreatic tissue
Islets of Langerhans damaged → insulin production reduced/stopped → Type 1 Diabetes
Main symptoms of CF
(respiratory system)
lung infection
Breathing difficulties
Main symptoms of CF
(reproductive system)
Infertility
Women → mucus blocks cervix, delays puberty, pregnancy still possible
Men → Many do not have a sperm duct, if present its typically blocked by mucus, very few can reproduce naturally
Main symptoms of CF
(sweat )
Very salty sweat
No functional CFTR protein so Cl⁻ & Na⁺ are not reabsorbed and instead are lost in sweat
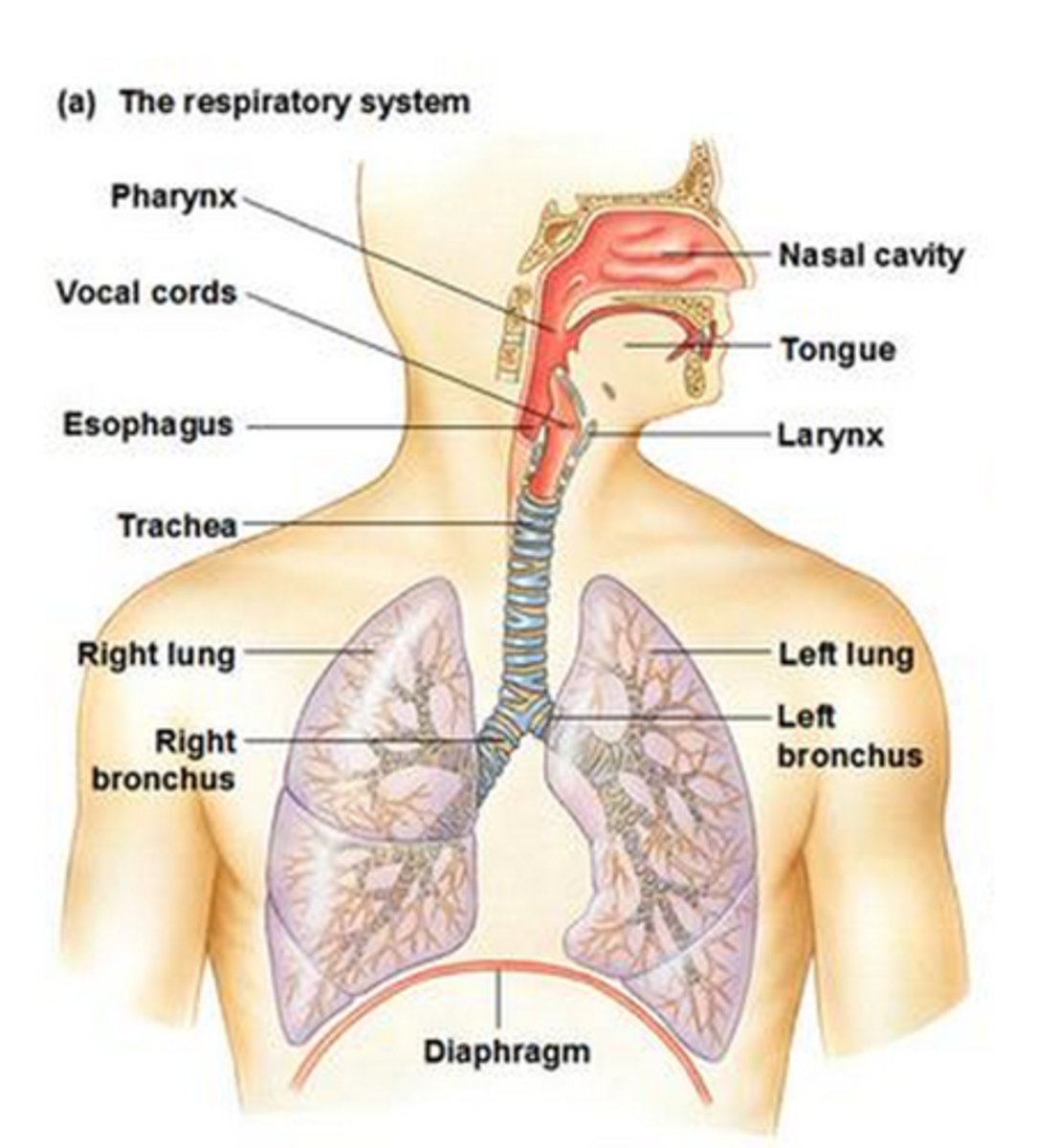
What's involved in the respiratory system
nasal cavity
nostrils
oral cavity
larynx
trachea
bronchus
lungs
bronchioles
alveoli
diaphragm
Goblet cells - adaptations + role
Produce mucus
Located between ciliated epithelia cells
Adaptations:
Large ER & Golgi
Many vesicles from which mucus is secreted out of cell
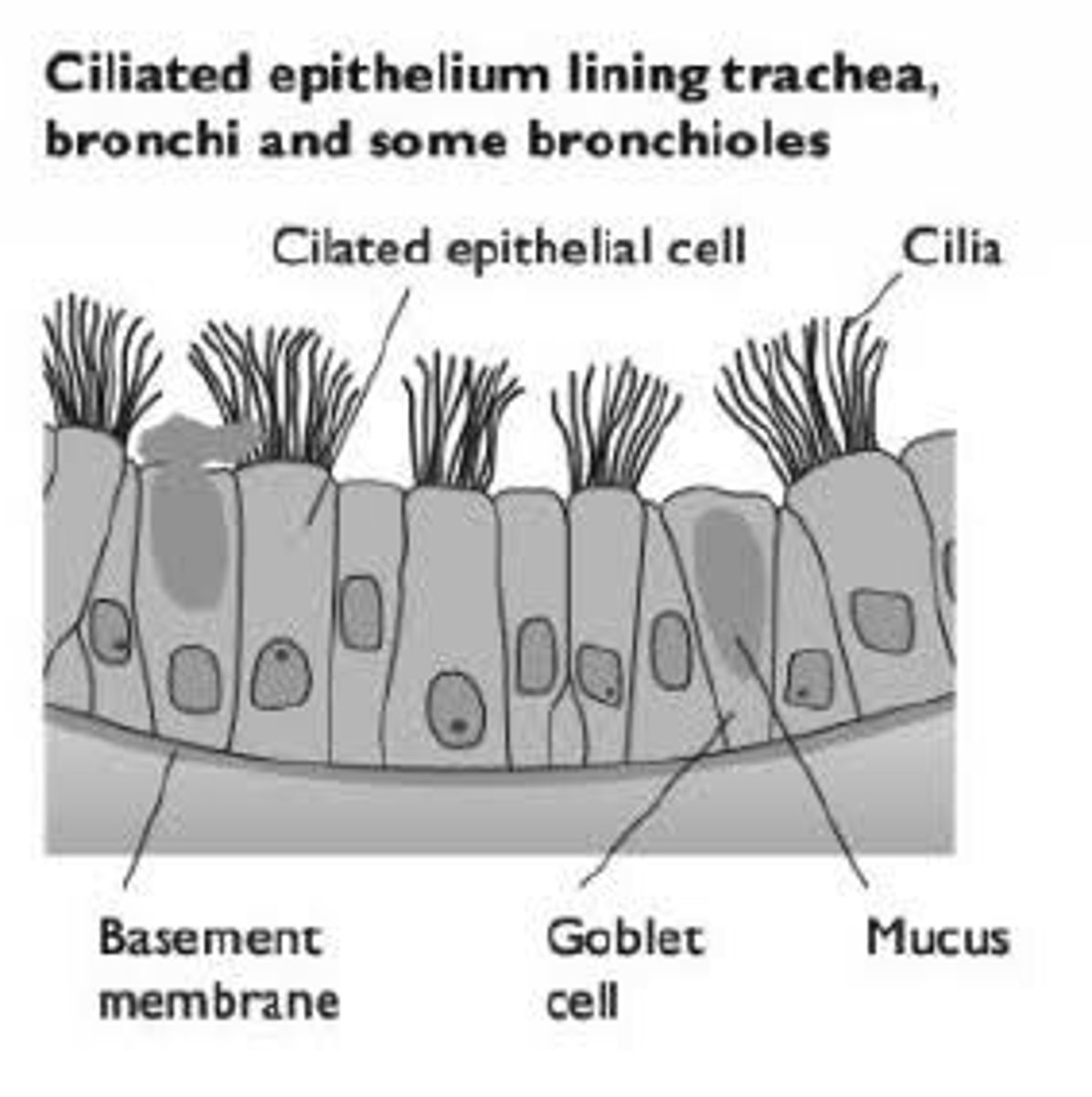
What is the role of mucus
To trap foreign particles (dust, bacteria) and remove them from entering the alveoli
Moisturises inhaled air
Type of epithelial cell & where they're located in the respiratory system (3)
Alveoli - simple squamous
Bronchioles - simple columnar
Trachea + Bronchi - Pseudostratified
Why do lung infections happen a lot
Mucus traps bacteria
Mucus is too sticky to be moved by cilia
Low O₂ levels in mucus, anaerobic bacteria thrive
WBC fighting bacteria die + release DNA making mucus stickier
Inflammation & infection
Mucus and breathing
Mucus blocks bronchioles
Less or no air reaches the alveoli
Reduced gas exchange
Diffusion
The movement of particles from an area of high concentration to an area of low concentration (down a concentration gradient) without using energy
Factors affecting diffusion
1. Concentration gradient
2. Surface area
3. Diffusion distance
4. Temperature
5. Size of molecule
Fick's law of diffusion
Rate of diffusion is inversely proportional to the thickness of the gas exchange surface
Why is gas exchange at alveoli efficient
Short diffusion distance - diffuses through 2 thin cells (epithelium & endothelium) to reach RBC + capillaries are one cell thick
Steep concentration gradient
Large surface area - many alveoli + they're really small → increases SA enormously
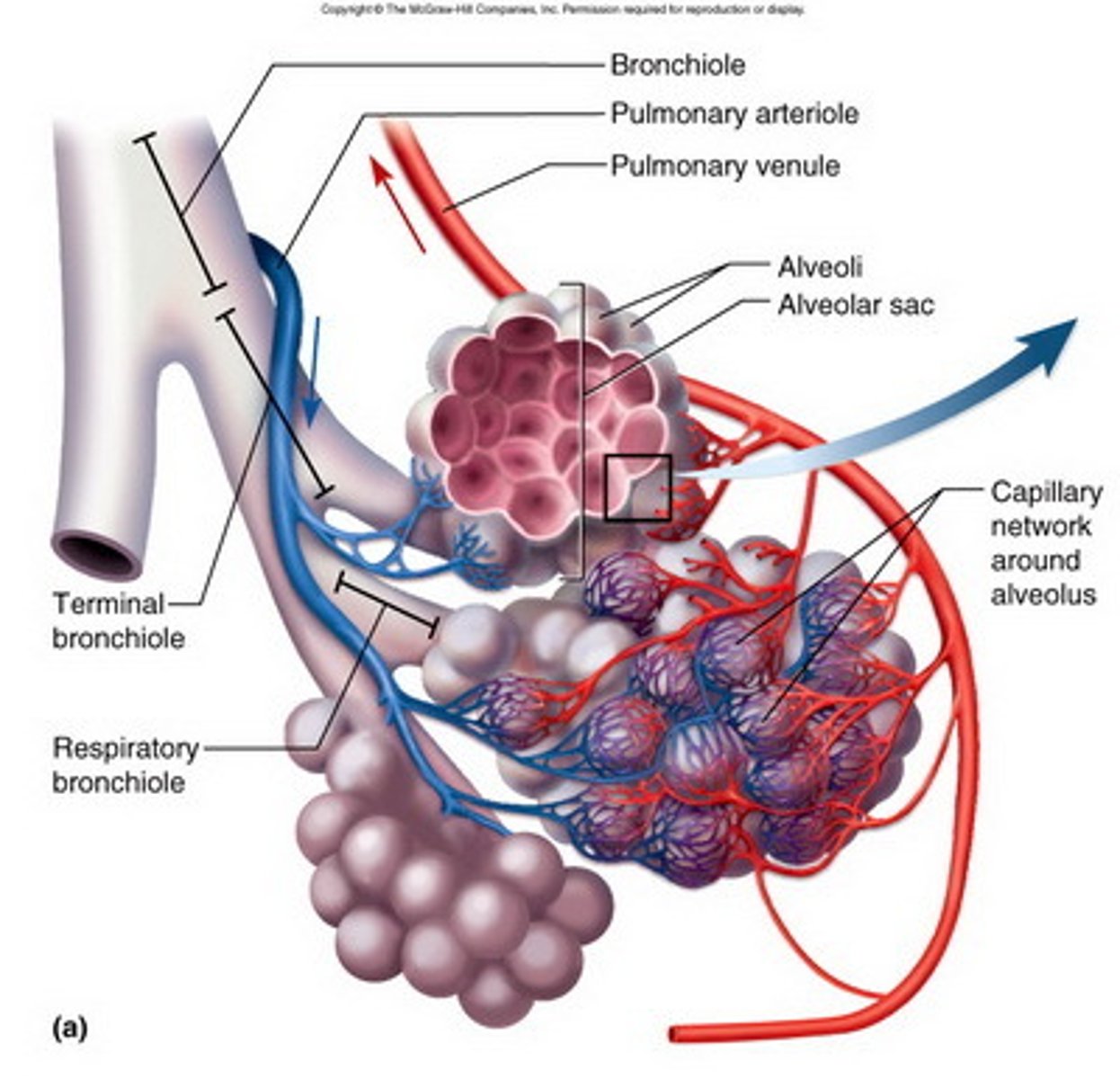
What is surfactant + role
Phospholipid
Prevents permanent collapse and sticking together of alveoli
How does build up of mucus in the bronchioles cause difficulties
Mucus blocks bronchioles → fewer alveoli available for gas exchange → reduce SA
Mucus fills alveoli → increased diffusion distance
Over-inflation of lungs tryna get enough O₂ → elasticity of lungs decrease → reduce gas exchange → O₂ shortage + tiredness
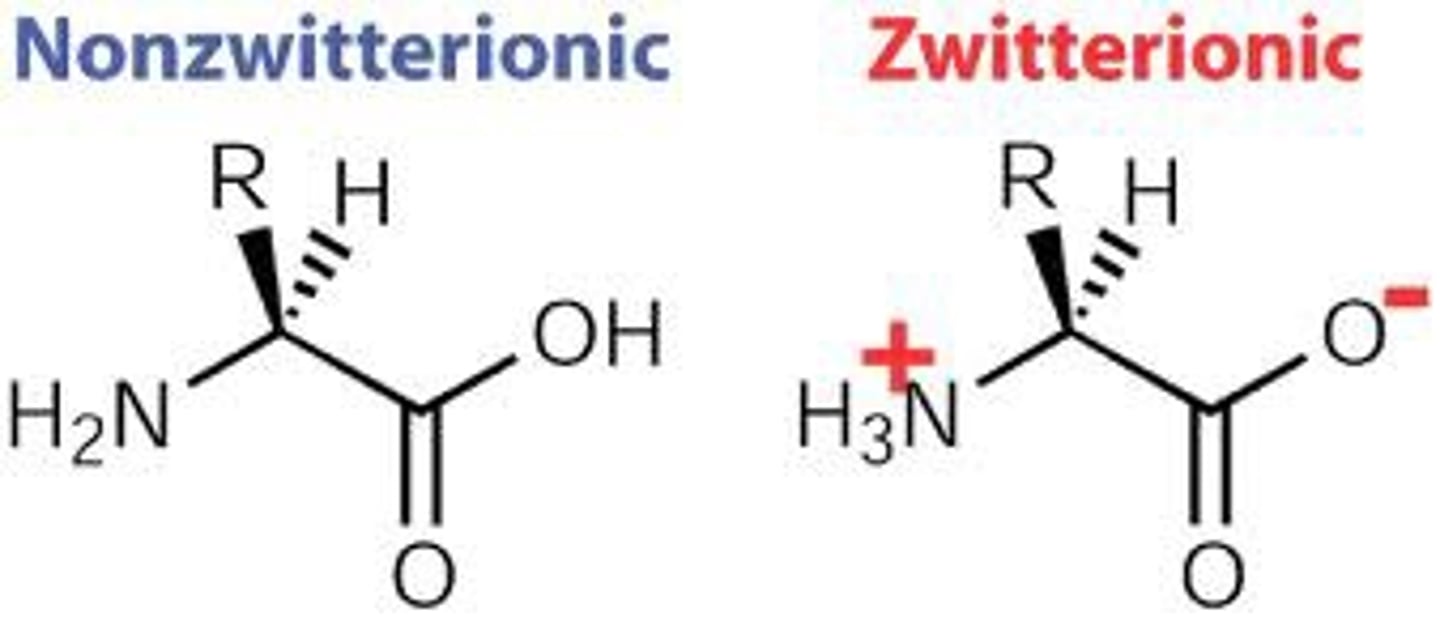
Zwitterions
In water at neutral pH, group are ionised
Dipeptide formation
Made via condensation reaction of two amino acids, form a peptide bond (amide bond), makes + removes a water molecule
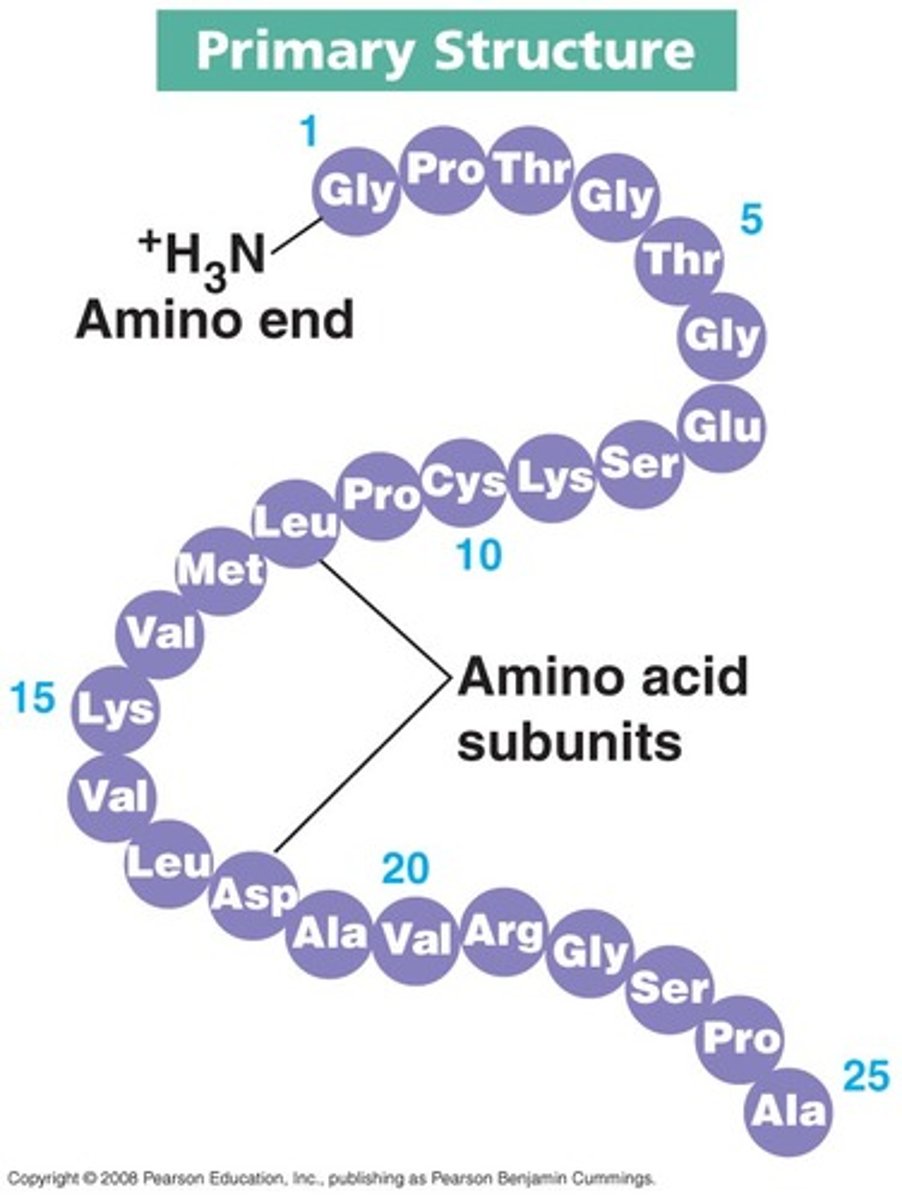
Primary structure
The linear sequence of the amino acid in a protein
- contains a N terminus and a C terminus
Secondary structure
The regular folding of parts of a protein due to hydrogen bonds between peptide bonds
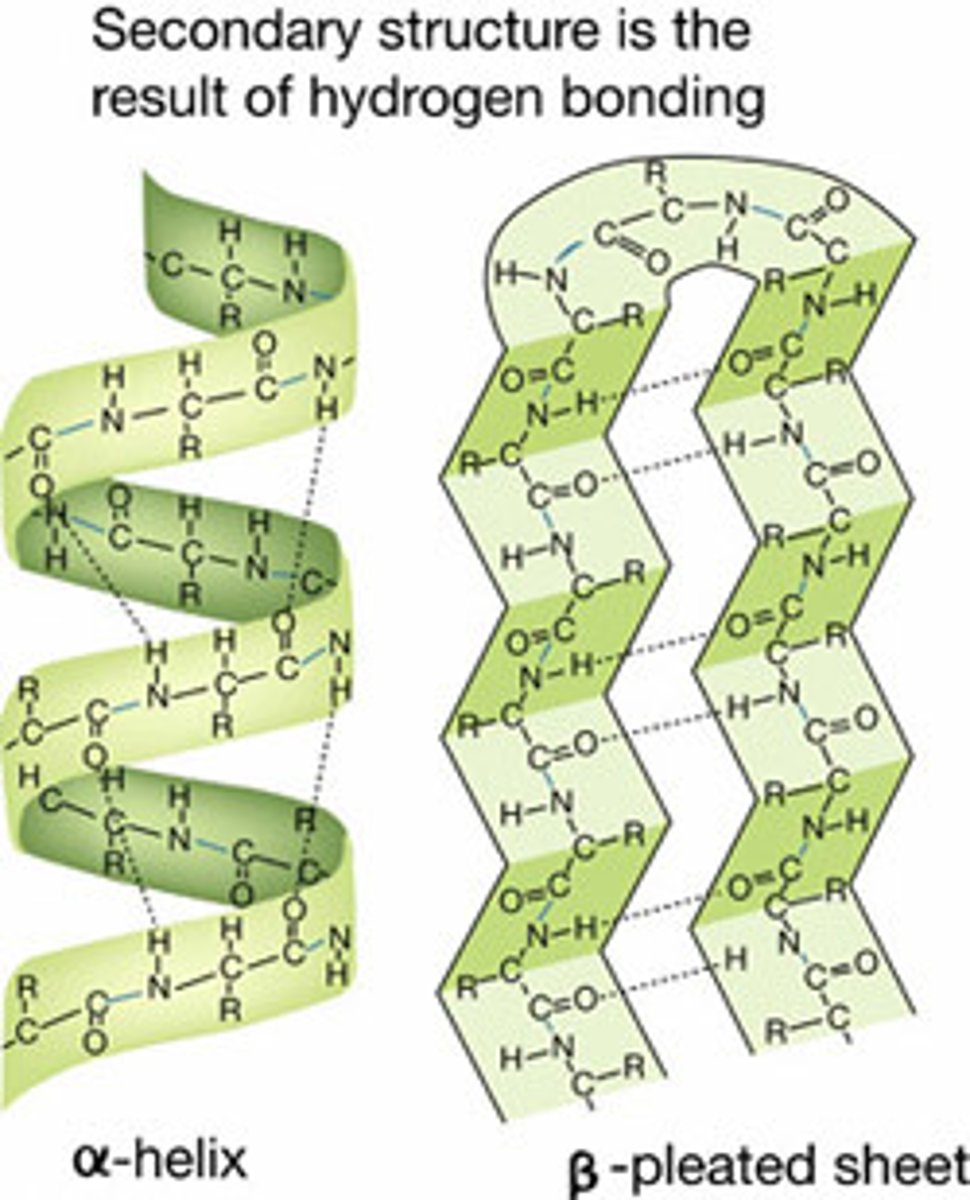
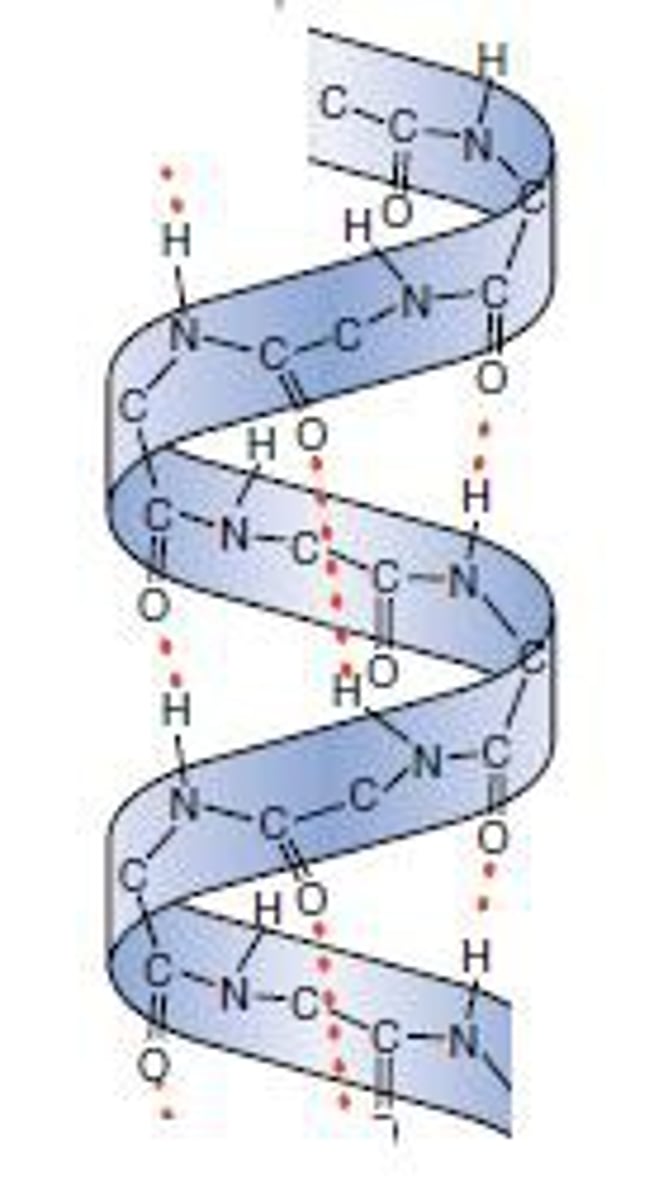
α helix
Forms a helix held by hydrogen bonds running PARALLEL with the long helical axis
Many hydrogen bonds makes it very stable & strong
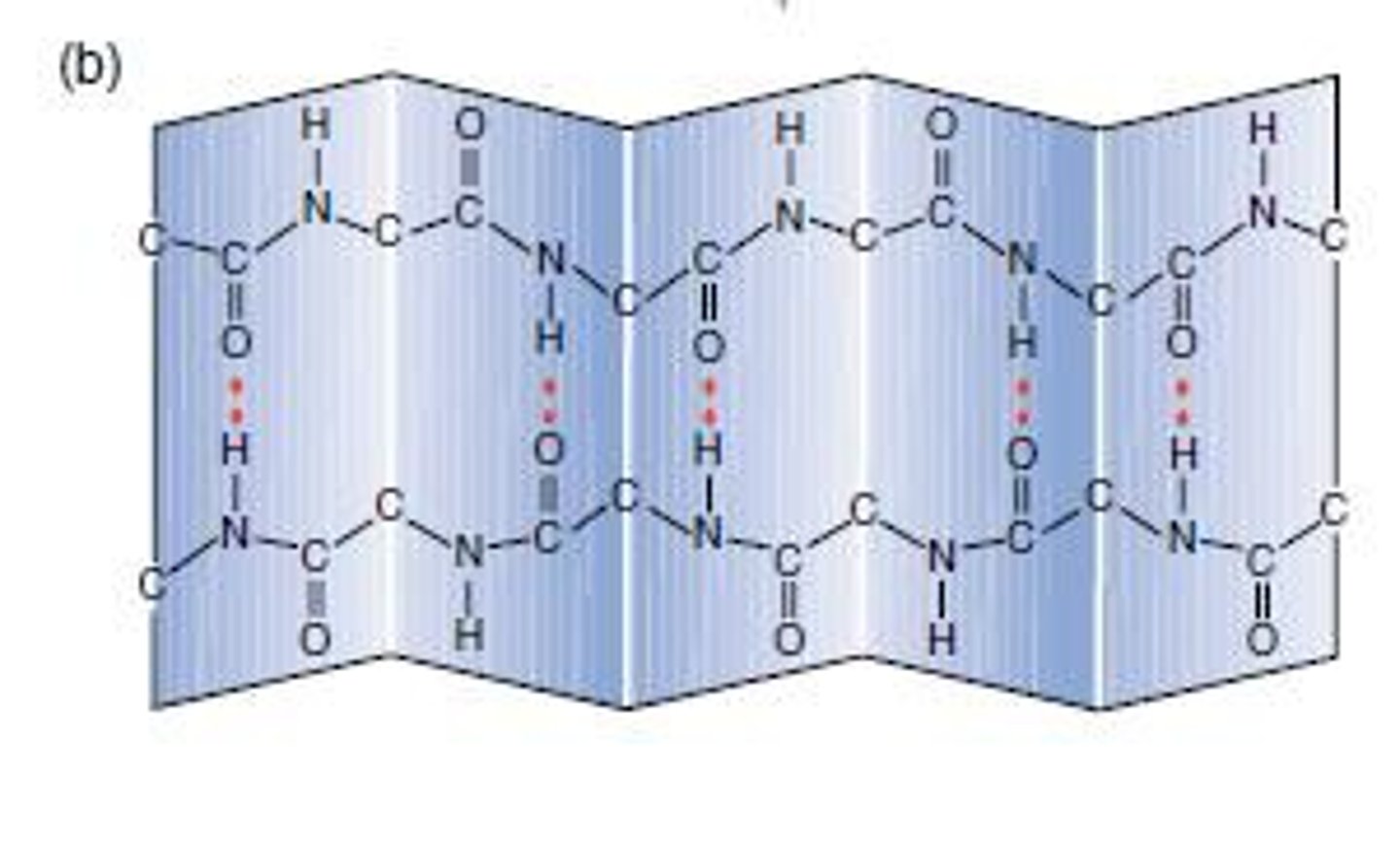
β pleated sheet
Chain zig-zags back and forward forming a sheet of antiparallel strands
Strands held together by hydrogen bonds between peptide bonds
Tertiary structure
Overall 3-dimensional structure of the whole peptide chain, formed by bonds between R group
Hydrophobic interactions - between R groups
Hydrogen bonds - between H & O of R groups
Ionic bonds - between charged R groups
Disulphide bonds - covalent bonds between two cysteine R groups
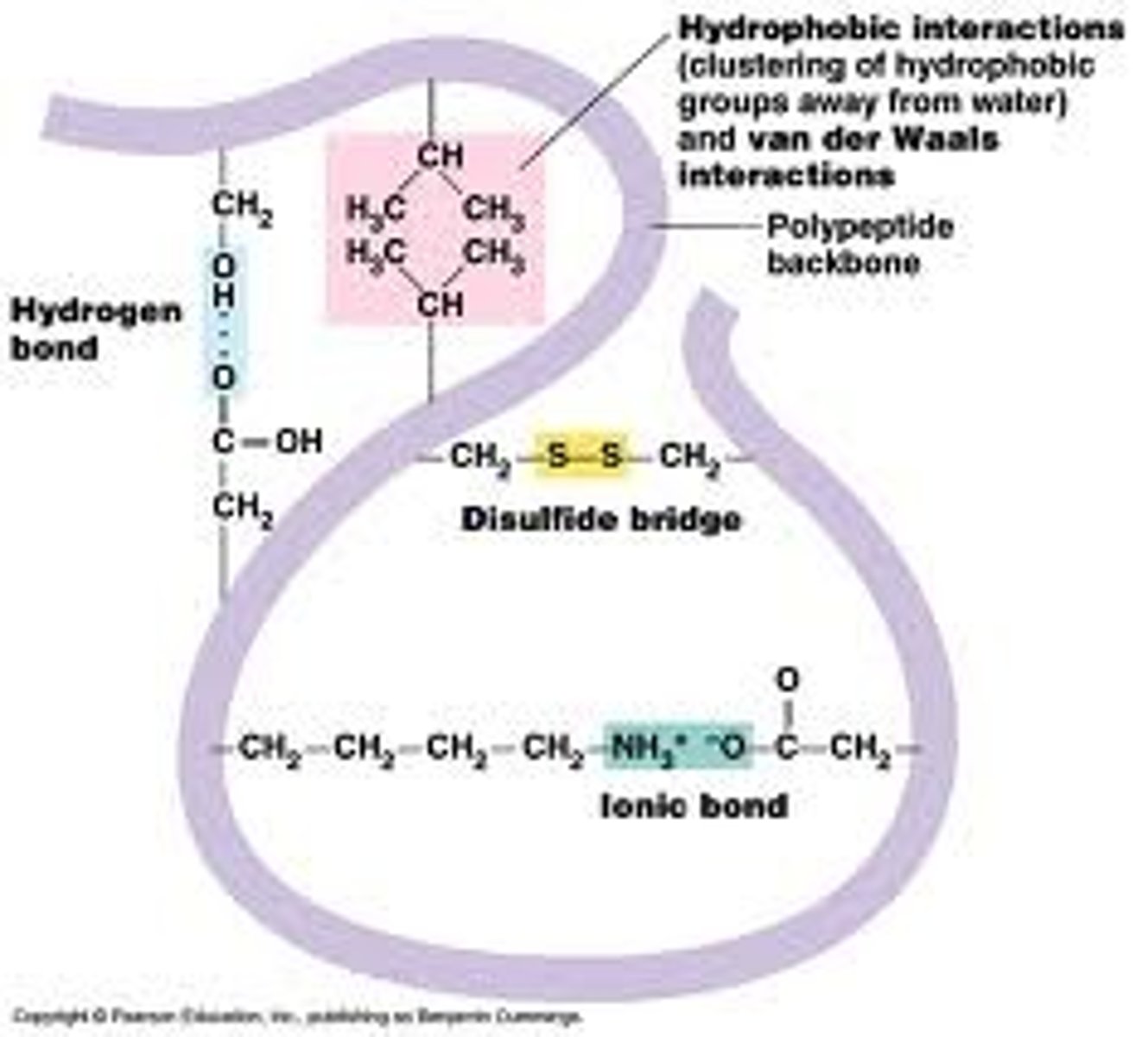
Quaternary structure
3-dimensional structure of several polypeptide chains interacting with each other via bonds between R groups
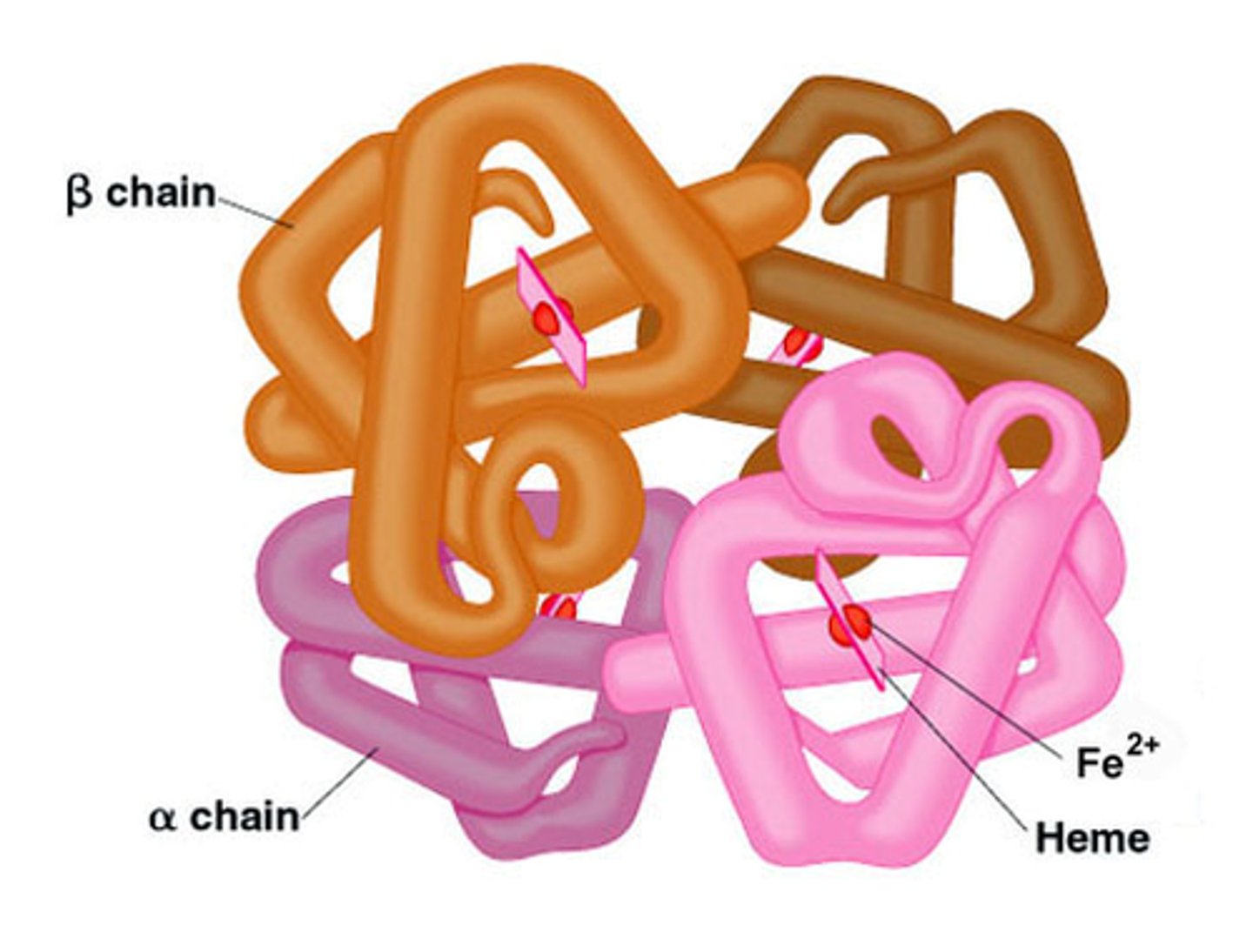
Conjugated proteins
Proteins that joined to other non protein molecules (prosthetic groups
Globular proteins - properties + role
Compact + roughly spherical
Non-polar hydrophobic R groups are orientated towards the centre
Polar hydrophilic R groups orientate themselves on the outside of the protein
Some contain a prosthetic group
Physiological roles: enzymes, antibodies, hormones
Fibrous proteins - properties + role
Long, strong strands
Little to no 3° structure
Highly repetitive sequences - very organised structures
Structural roles: collagen & keratin
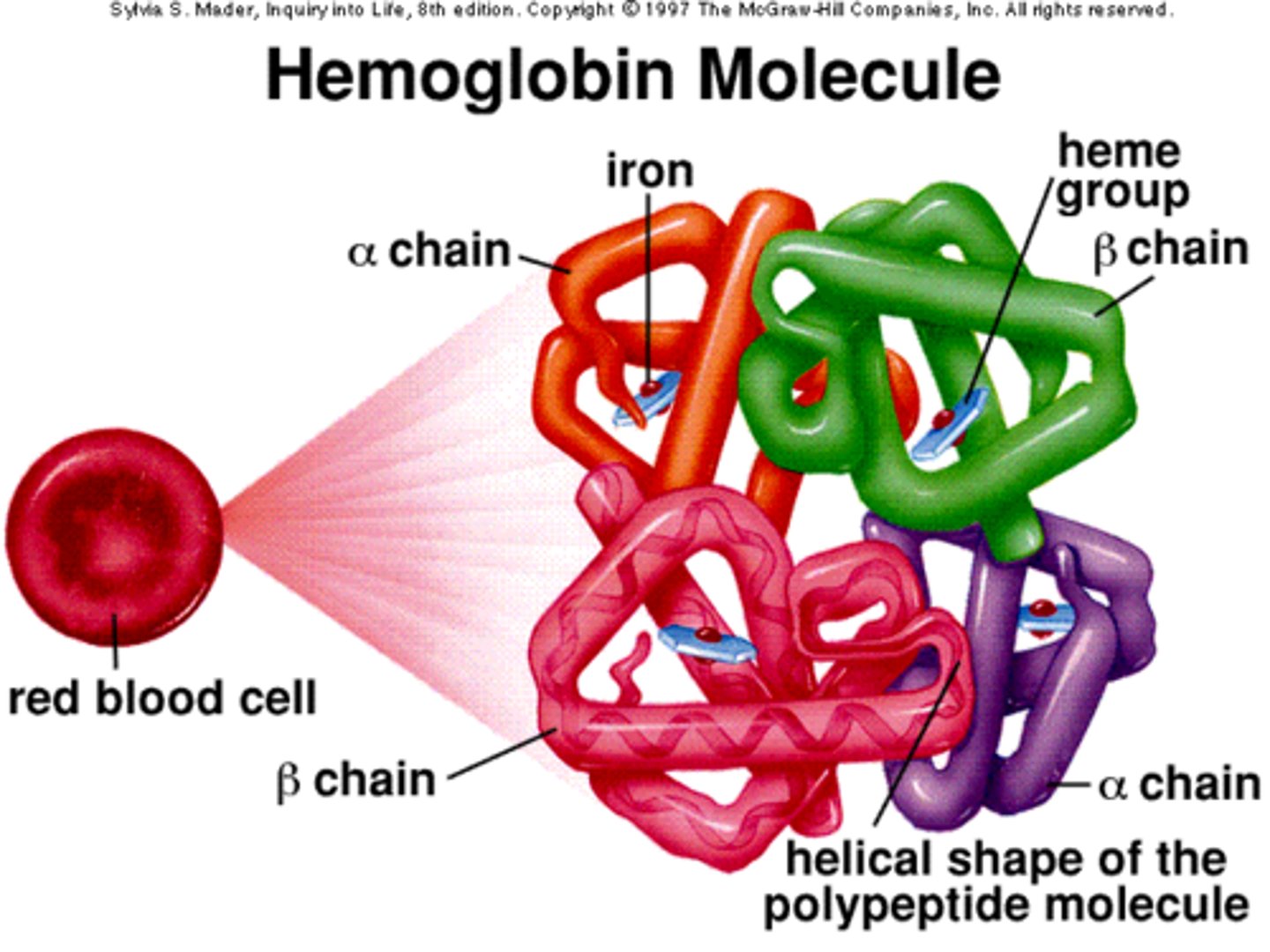
Haemoglobin - properties + role
4 polypeptide chains - 2x α -globin & 2x β - globin
Spherical, globular
Variable AA
Prosthetic - Haem group
Soluble in H₂O
Function - transporting O₂
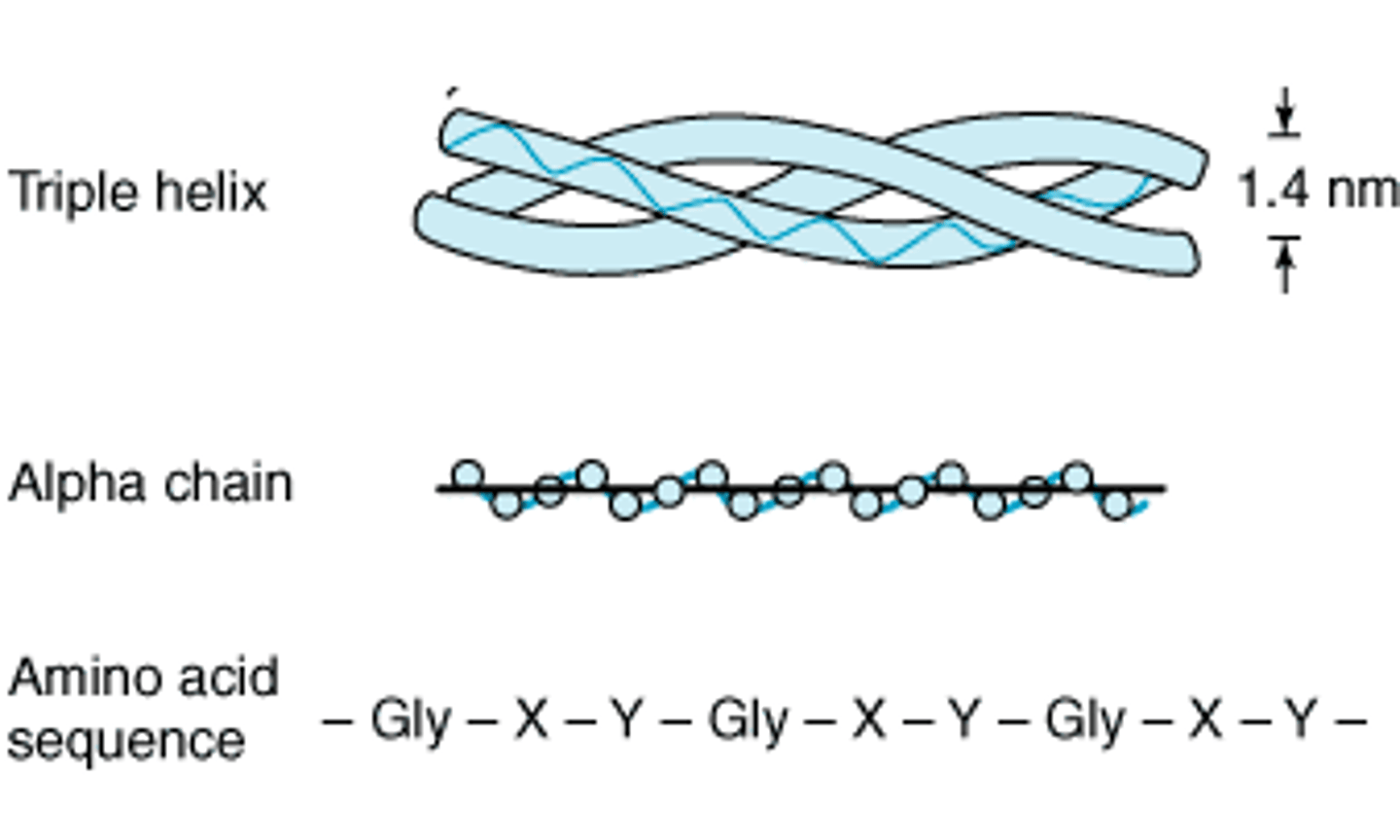
Collagen - properties + role
3 polypeptide chains - triple helix
Long, thin, fibrous
Repetitive AA -every 3rd AA is glycine
No prosthetic group
Insoluble in H₂O
Function - connective tissue e.g. tendons, skin
Gorter and Grendel model - conclusion
Phospholipids form a bilayer
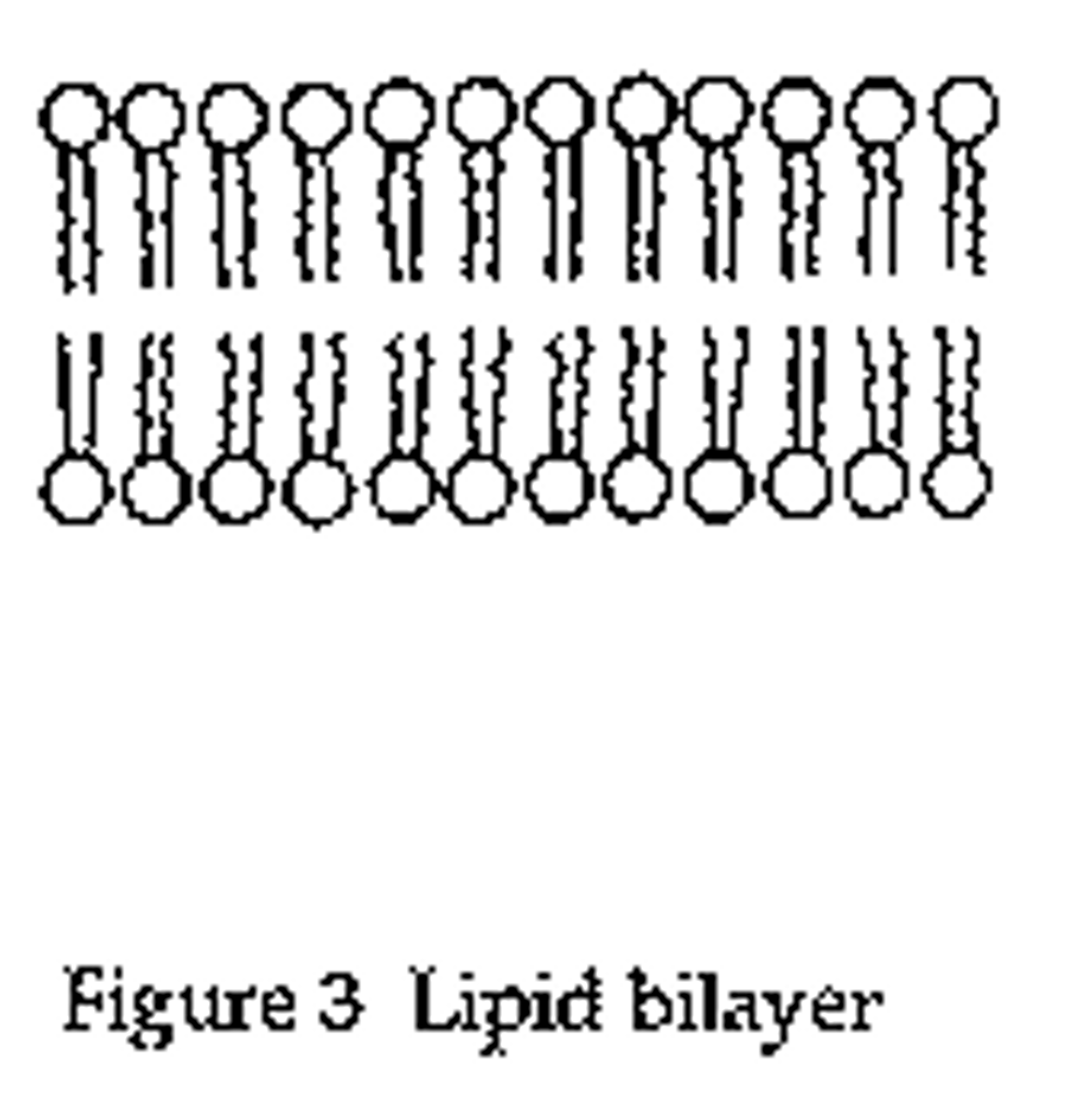
Gorter and Grendel model - evidence
Number of phospholipids extracted from red blood cell membranes = double the area of the plasma membrane if it was arranged as a monolayer
Gorter and Grendel model - problems
Did not explain the location of proteins or how insoluble molecules were moved in & out the cell
Davson and Danielli's model - conclusion
Proteins hold lipid bilayer in place & 'Protein lipid sandwich' model
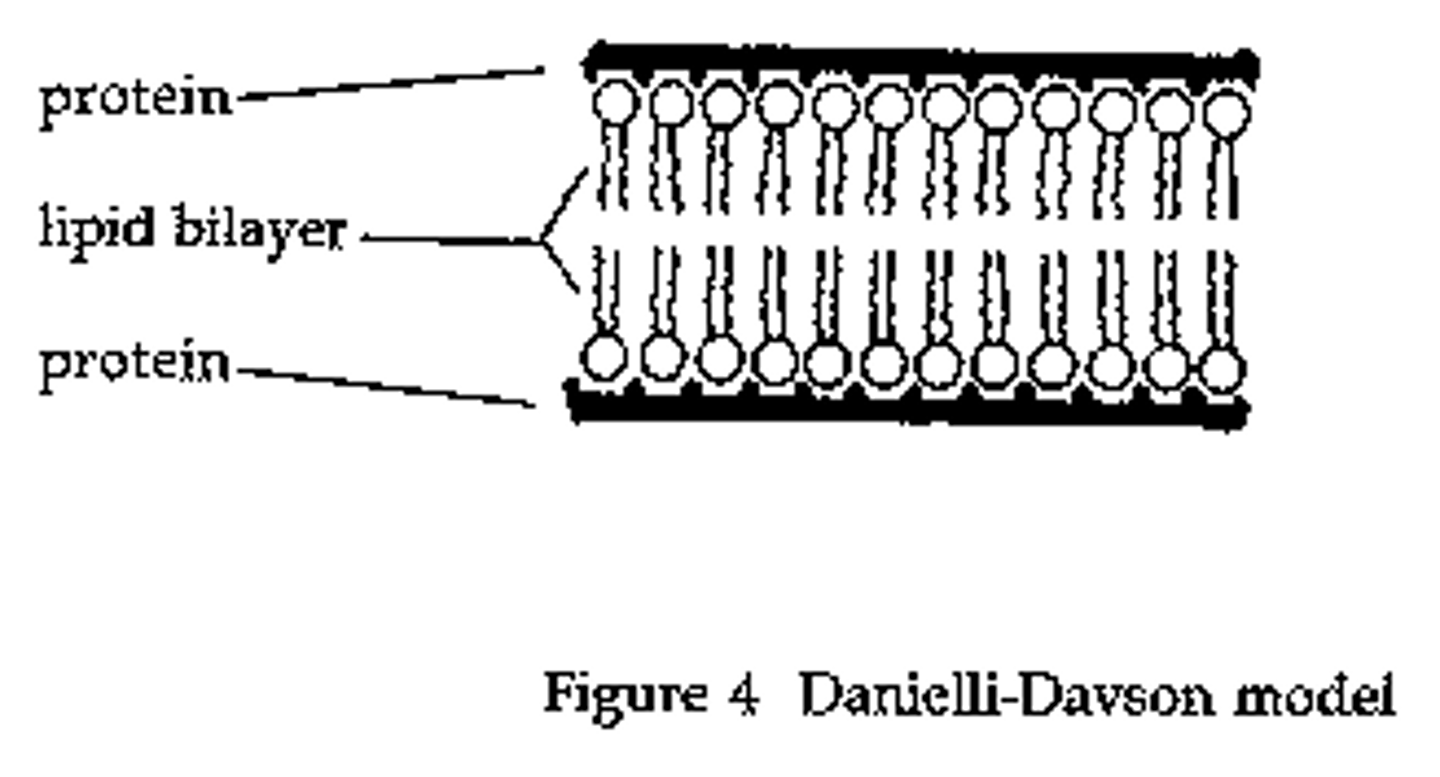
Davson and Danielli's model - evidence
Membranes effective at controlling the movement of substances in & out of cells.
Electron micrographs showed the membrane had two dark lines with a lighter band between (In electron micrographs, proteins appear darker than phospholipids)
Davson and Danielli's model - problems
Freeze etched electron micrographs of the centre of the membrane showed globular structures scattered throughout.
Improvements in technology used to analyse the proteins in the membranes showed that proteins were globular, varied in size and had parts that were hydrophobic
Singer and Nicolson model - conclusion
FLUID MOSAIC MODEL
Lipids form two-dimensional liquid in which lipids can move (fluid)
Proteins attach to membrane some outside, some embedded within (mosaic)
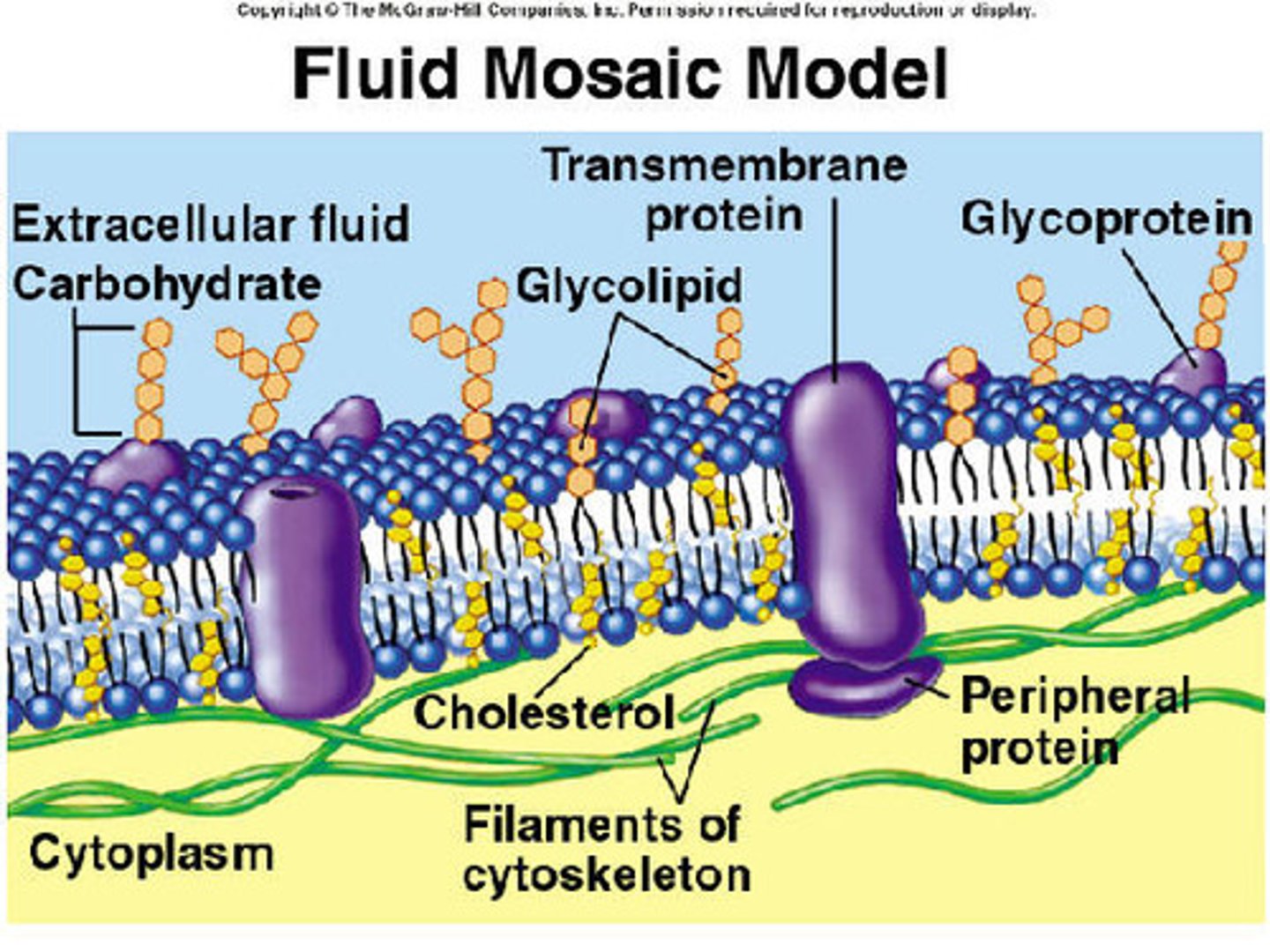
Fluid
Two-dimensional 'liquid' with lateral movement of lipids and proteins through bilayer
Mosaic
Composed of different types of macromolecules
e.g. integral & peripheral proteins, lipids and glycolipids etc
Singer and Nicolson model - evidence
Analysis of freeze-etched electron micrographs showed proteins extending into the centre of membranes
Biochemical analysis of the plasma membrane components showed that membrane proteins are free to move within the bilayer
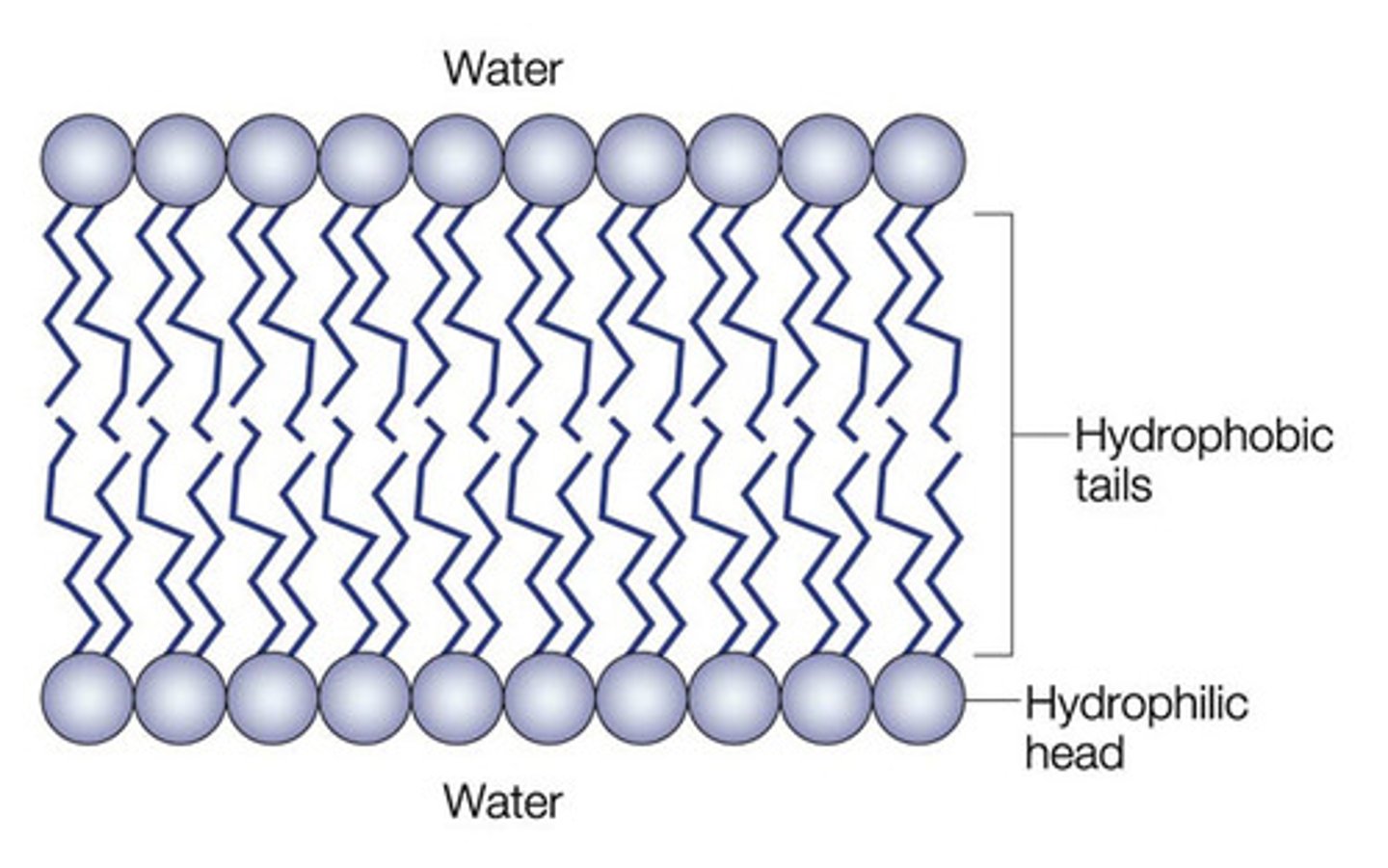
Phospholipid bilayer
Polar, hydrophilic, phosphate heads face towards solution
Non polar, hydrophobic lipid tail faces away from the solution
A bilayer forms with hydrophobic parts pointing in towards the centre of the bilayer/towards each other
Unsaturated fatty acids in the cell membrane
Causes the phospholipid to be less tightly packed
More movement possible
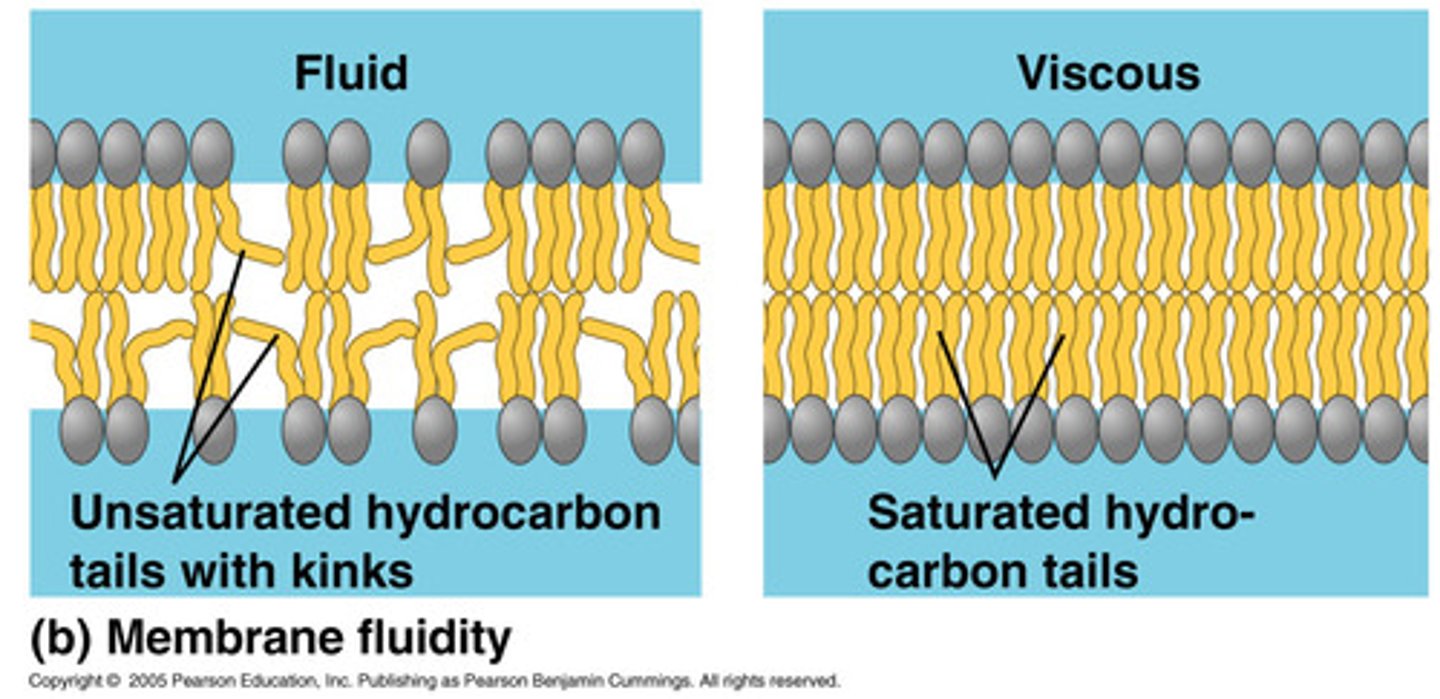
Role of cholesterol in the cell membrane
Regulates fluidity,
- prevents phospholipids from packing too closely together when temps low
- stabilises the cell membrane at higher temperatures
Role of glycolipids & glycoproteins
Markers for cell-to-cell recognition
Signalling
How do non polar molecules move through the membrane
Diffusion
Facilitated diffusion
Movement of molecules from an area where they are at a high concentration to an area where they are at low concentration via a carrier or channel protein
What are channel proteins used to transport
Polar molecules e.g. ions
What are carrier proteins used to transport
Larger molecules e.g. glucose or amino acids
Osmosis
Movement of water molecules from a high water potential to a low water potential across a selectively-permeable membrane
OR
Net movement of water into areas of high solute concentration across a selectively permeable membrane
Osmosis in animal cells
(using different solutions)
Hypotonic - solution with lower [solute] than cell / cell burst (lysed)
Isotonic - solution with same [solute] as cell / cell stays normal
Hypertonic - solution with greater [solute] than cell / cell shrivels
![<p>Hypotonic - solution with lower [solute] than cell / cell burst (lysed)</p><p>Isotonic - solution with same [solute] as cell / cell stays normal</p><p>Hypertonic - solution with greater [solute] than cell / cell shrivels</p>](https://knowt-user-attachments.s3.amazonaws.com/1599160d-de18-42e3-b180-26dd586a7965.jpg)
Osmosis in plant cells
(using different solutions)
Hypotonic - cell goes turgid
Isotonic - cell goes flaccid
Hypertonic - plasmolysed
Active transport
Movement of molecules from a low concentration to a high concentration via carrier proteins using energy from ATP
Exocytosis
Export of molecules out of cells involving vesicles that fuse with the cell membrane and release their contents
Endocytosis
Uptake of molecules by the creation of a vesicle from the cell membrane
What happens when there is too little water in the mucus of a healthy person
1. Cl⁻ is pumped into cell from tissue fluid across basal membrane
2. CFTR channel is open causing Na⁺ channel to close; Cl⁻ diffuses through the open CFTR channel
3. Na⁺ diffuses down the electrical gradient into mucus
4. High [salt] in mucus draws water out of the cells into mucus by osmosis: mucus becomes more runny
5. Water is drawn into cells from tissue fluid by osmosis
![<p>1. Cl⁻ is pumped into cell from tissue fluid across basal membrane</p><p>2. CFTR channel is open causing Na⁺ channel to close; Cl⁻ diffuses through the open CFTR channel</p><p>3. Na⁺ diffuses down the electrical gradient into mucus</p><p>4. High [salt] in mucus draws water out of the cells into mucus by osmosis: mucus becomes more runny</p><p>5. Water is drawn into cells from tissue fluid by osmosis</p>](https://knowt-user-attachments.s3.amazonaws.com/cd55562c-171d-4137-a141-abedb8ecdf37.jpg)
Why is mucus so sticky in cystic fibrosis
1) CFTR is absent or non-functional
2) Na⁺ channel is permanently open and Na+ diffuses into cells from mucus, and is pumped into tissue fluid
3) Cl⁻ diffuses out of mucus towards basal membrane via the intercellular space down an electrical gradient
4) Water is continually removed from mucus by osmosis; mucus becomes very sticky
5) Bacteria and WBC get trapped; DNA released from dead WBC makes mucus even more sticky
Lock and key theory
Substrate shape is complementary to shape of active site
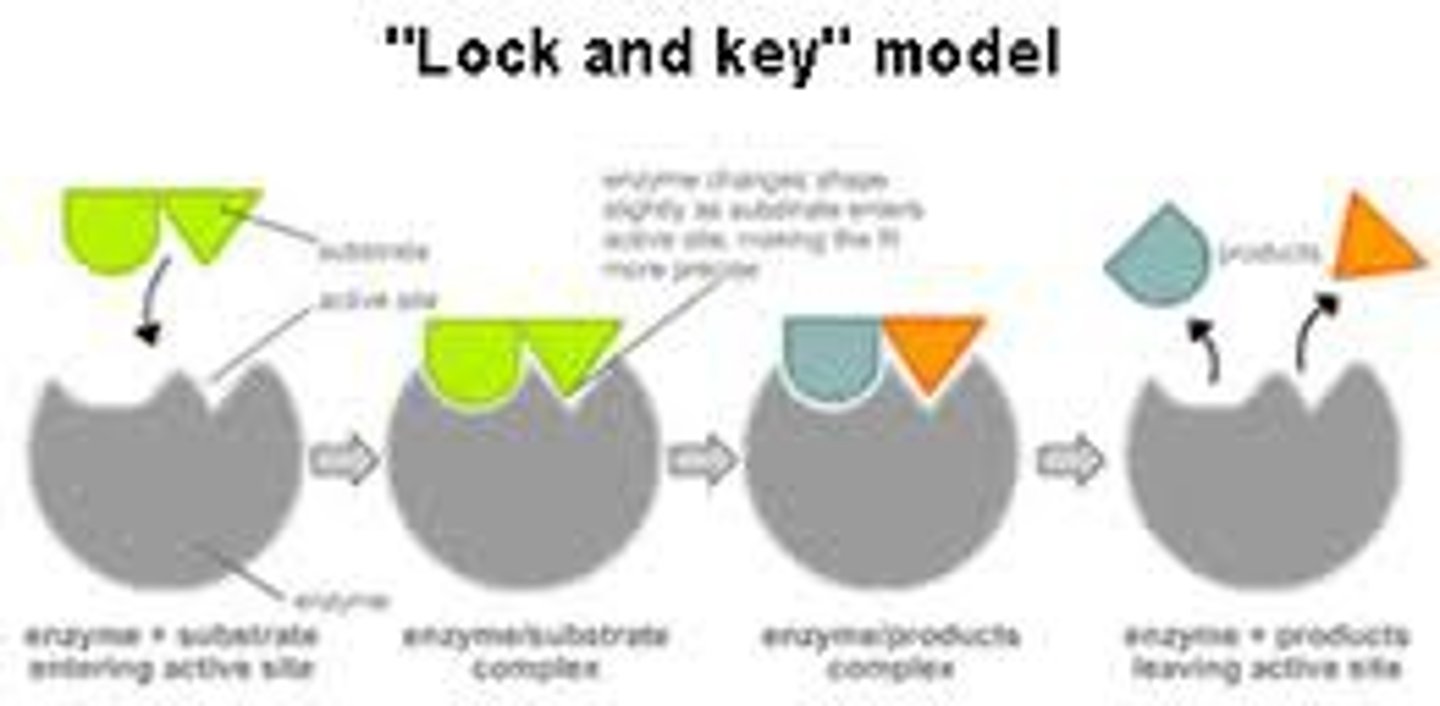
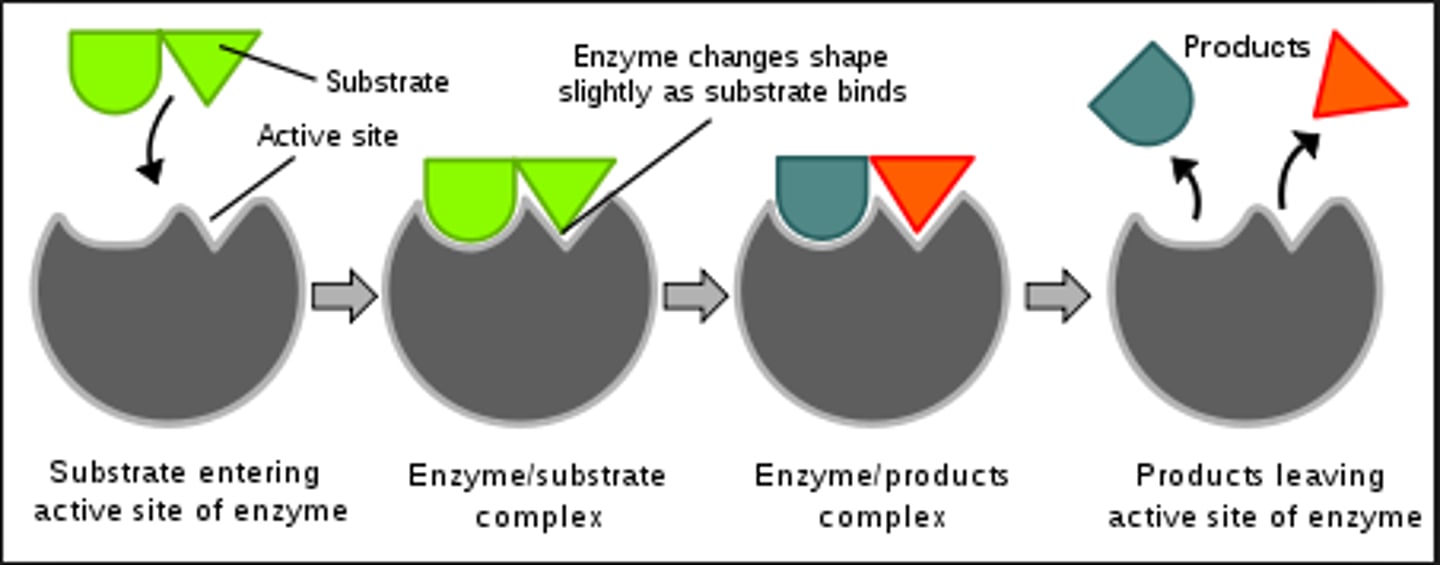
Induced fit theory
Substrate binding induces shape change in active site which enables perfect fit and catalytic activity
Enzyme
Globular proteins that act as biological catalyst and speed up chemical reactions
They lower activation energy
What factors affect enzyme activity
Temperature
pH
[Substrate]
[Enzyme]
Inhibitors
Cofactors
Cofactors
Non-protein chemical compounds that are bound to enzymes and required for enzyme activity
Coenzyme - loosely bound cofactor (e.g. vitamin c, metal ions)
Prosthetic group - tightly bound cofactor (e.g. haem group)
Enzyme inhibitors
Competitive inhibitors - competes with substrate for active site ( can overcome by increasing [substrate] )
Non-competitive inhibitors - binds to allosteric site and changes shape of active site
Pyrimidines
1 carbon ring
Thymine & Cytosine
Purines
2 carbon rings
Adenine & Guanine ';
Facts about DNA
Codes for AA sequence in proteins
Deoxyribose sugar; A,C,G,T bases; double strand; very long
Located in nucleus
Constant amount in cells
Chemically very stable
Facts about RNA
mRNA (transcription); tRNA (translation)
Ribose sugar; A,C,G,U bases; single strand; shorter strands
Made in nucleus, found throughout cell
Varying amount in cells
Chemically unstable
Transcription
Copying of a length of DNA code one gene long into messenger RNA (mRNA)
- done in the nucleus
- catalysed by RNA polymerase
1.1 Transcription initiation (STEP 1)
1. RNA polymerase bind to DNA and scans for promoter region
2. RNA polymerase partially unwinds the DNA double helix
3. RNA polymerase starts to transcribe DNA sequence on the anti-sense strand into RNA sequence via complementary base pairing
Anti-sense strand transcribed
1.2 Transcription elongation
Transcription continues until the terminator is reached
1.3 Transcription termination
1. RNA polymerase reaches terminator
2. Transcription stops: RNA polymerase & RNA transcript are released
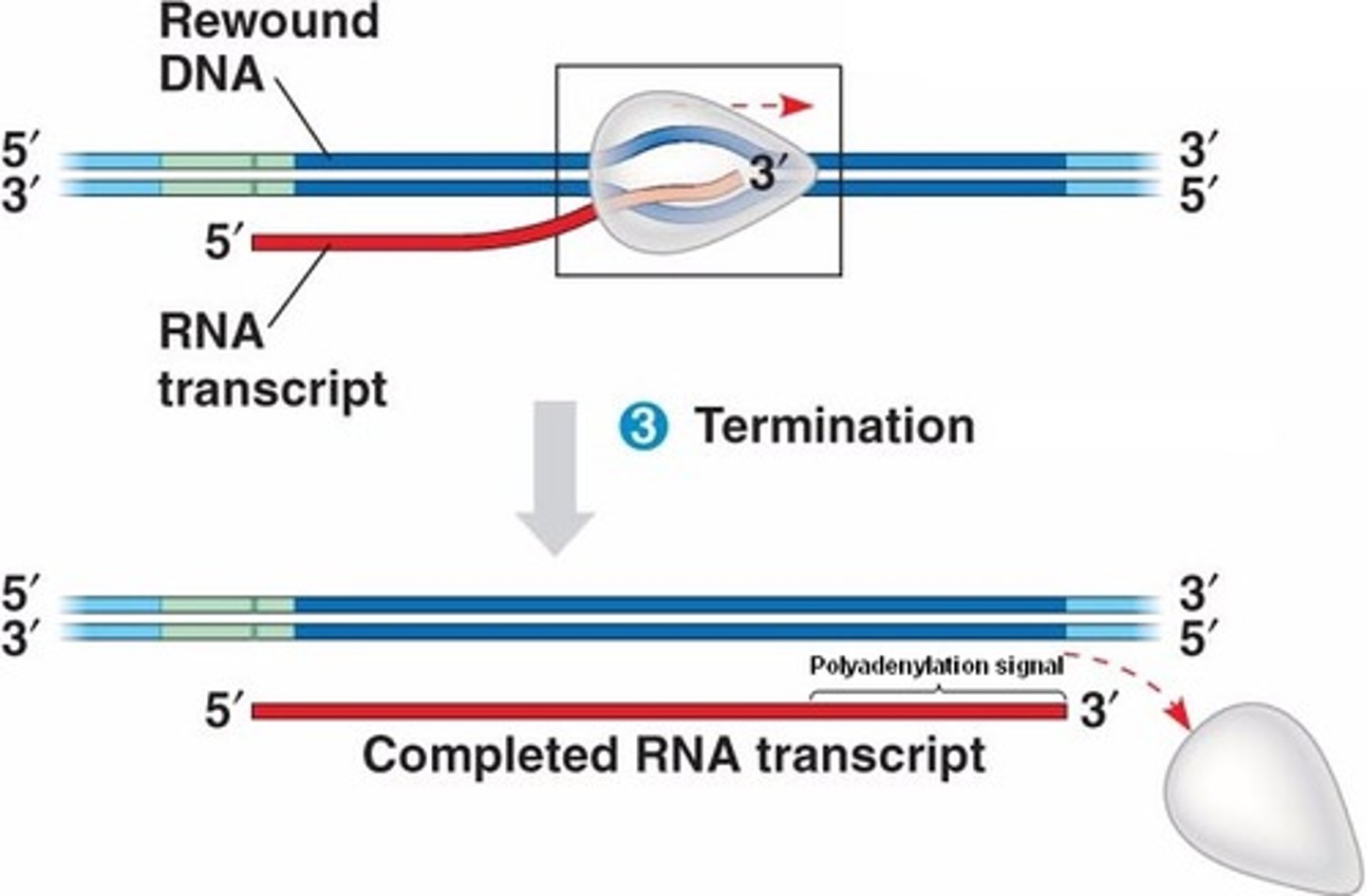
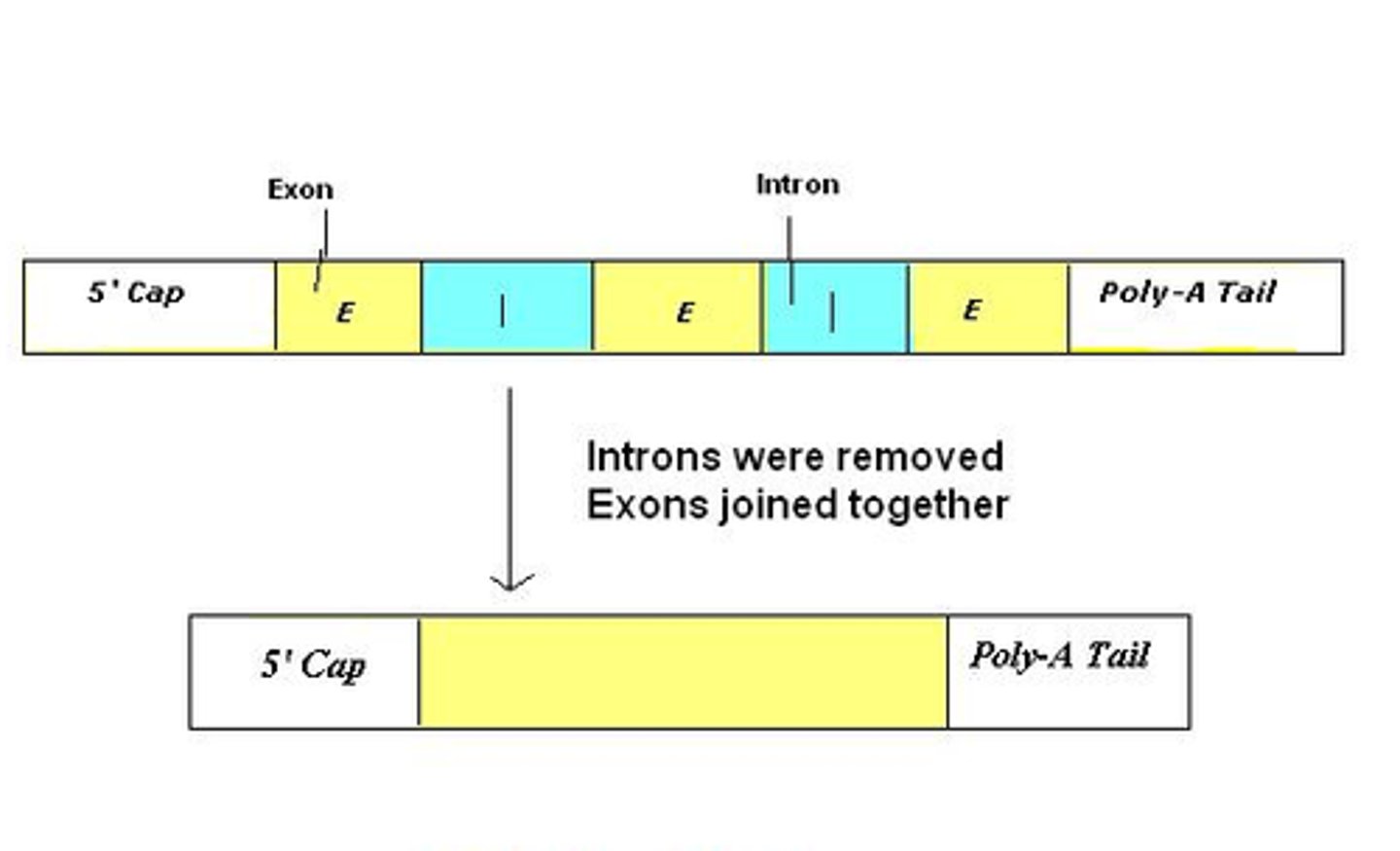
2.1 RNA processing
1. RNA transcript is spliced (introns, non-coding, are removed)
2. RNA transcript receives cap & tail
3. Processed RNA transcript = mRNA
3.1 mRNA export
mRNA is exported out of the nucleus via pore in the nuclear membrane
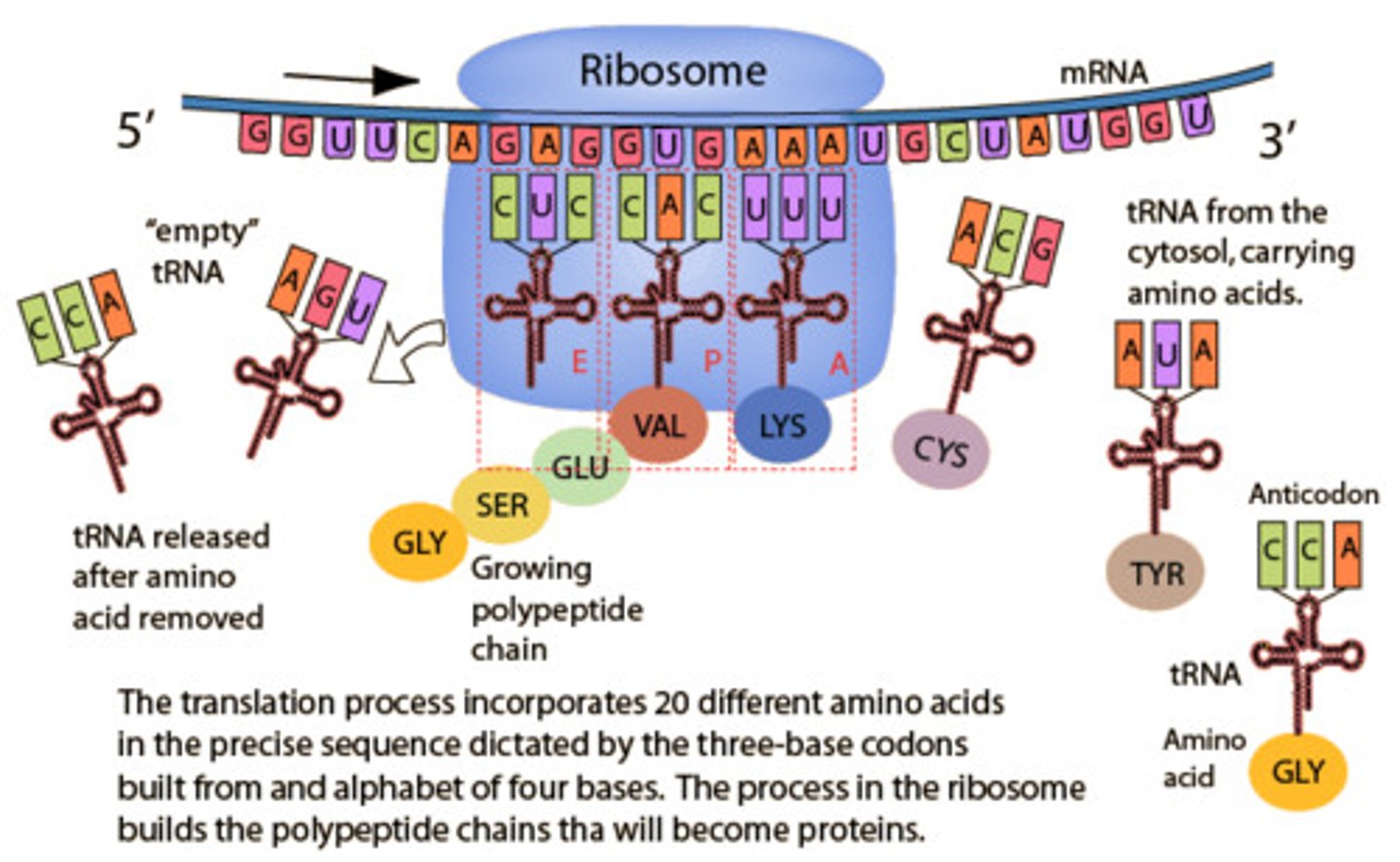
Translation process
1. Ribosome attaches to mRNA
2. Ribosome moves along mRNA (5' → 3') to find start codon
3. tRNA carrying specific AA w/ anticodons pairs up w/ codons (complimentary base pairing)
4. Next tRNA w/ anticodon complementary to next mRNA codon is loaded
5. Adjacent AAs joined via a peptide bond, catalysed by peptide synthetase
6. First tRNA is released
7. Elongation continues till stop codon reached
8. Release factor is loaded & translation stops
9. Polyprotein released
Nature of genetic code
Triplet code: 3 codons code for 1 AA
Degenerate: some AA's can be coded for by several codons
Universal: same code in all organisms
Non-overlapping: each codon is read separately
tRNA
Different tRNA for every codon
tRNA has anticodon sequence complementary to codon
tRNA binds to specific AA corresponding to anti-codon
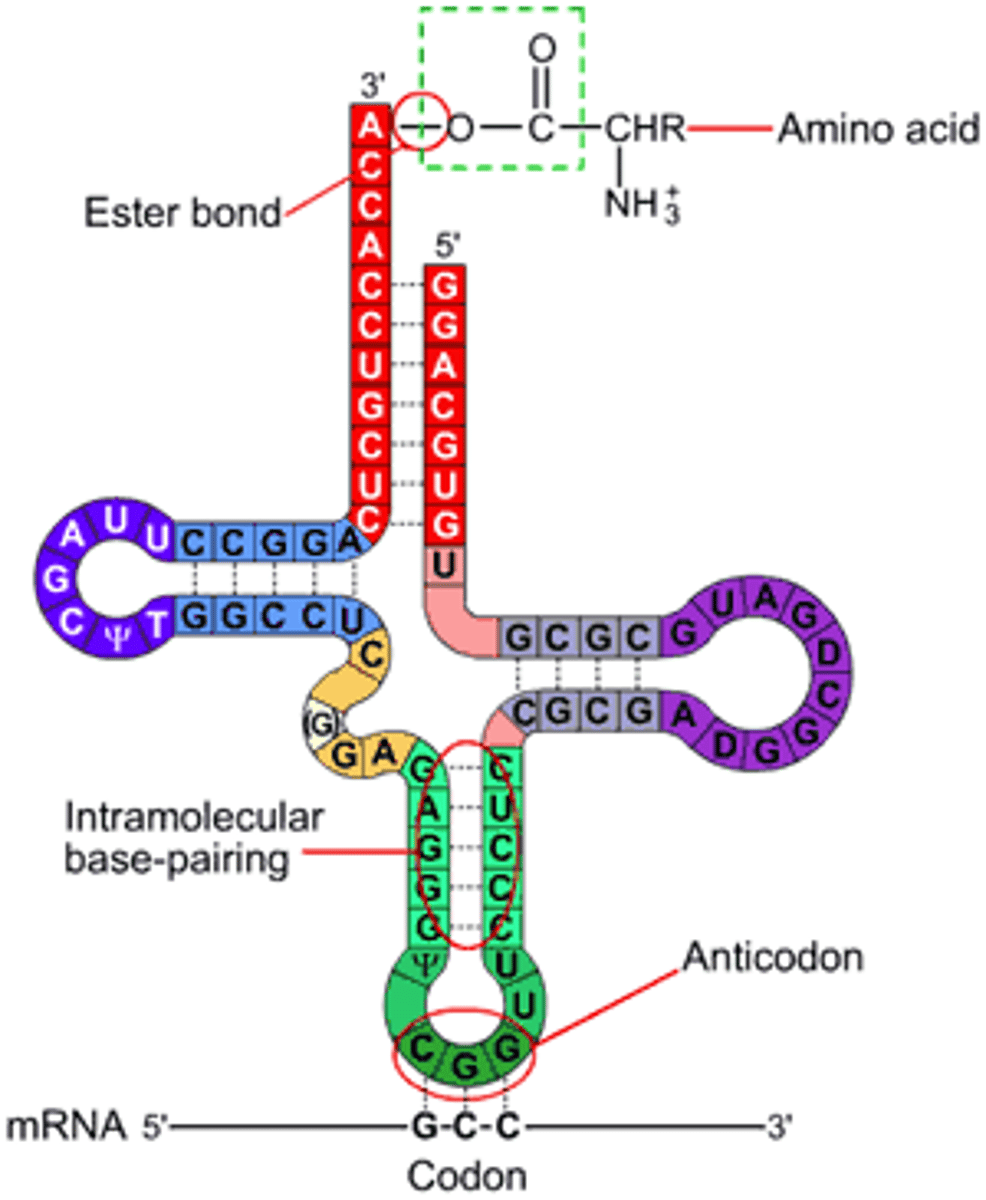
Translation
Using the sequence of codons on mRNA to produce a sequence of amino acids in a protein
- occurs in cytoplasm
- catalysed by ribosomes, tRNA & peptide synthetase
Polysomes
Several ribosomes can bind to the same mRNA forming a polysome
- several polypeptide chains are made simultaneously
DNA replication
Copying of the entire genome in preparation for cell division
Steps of DNA replication
1. DNA helicase unwinds DNA double helix
2. The two strands of the DNA double helix are separated by breaking hydrogen bonds between base pairs
3. Parent strands act as template for new DNA strands, free nucleotides pair with the complimentary nucleotides, DNA polymerase forms phosphodiester bonds
4. Two identical new strands of DNA are made
5. Newly formed DNA rewinds into helices
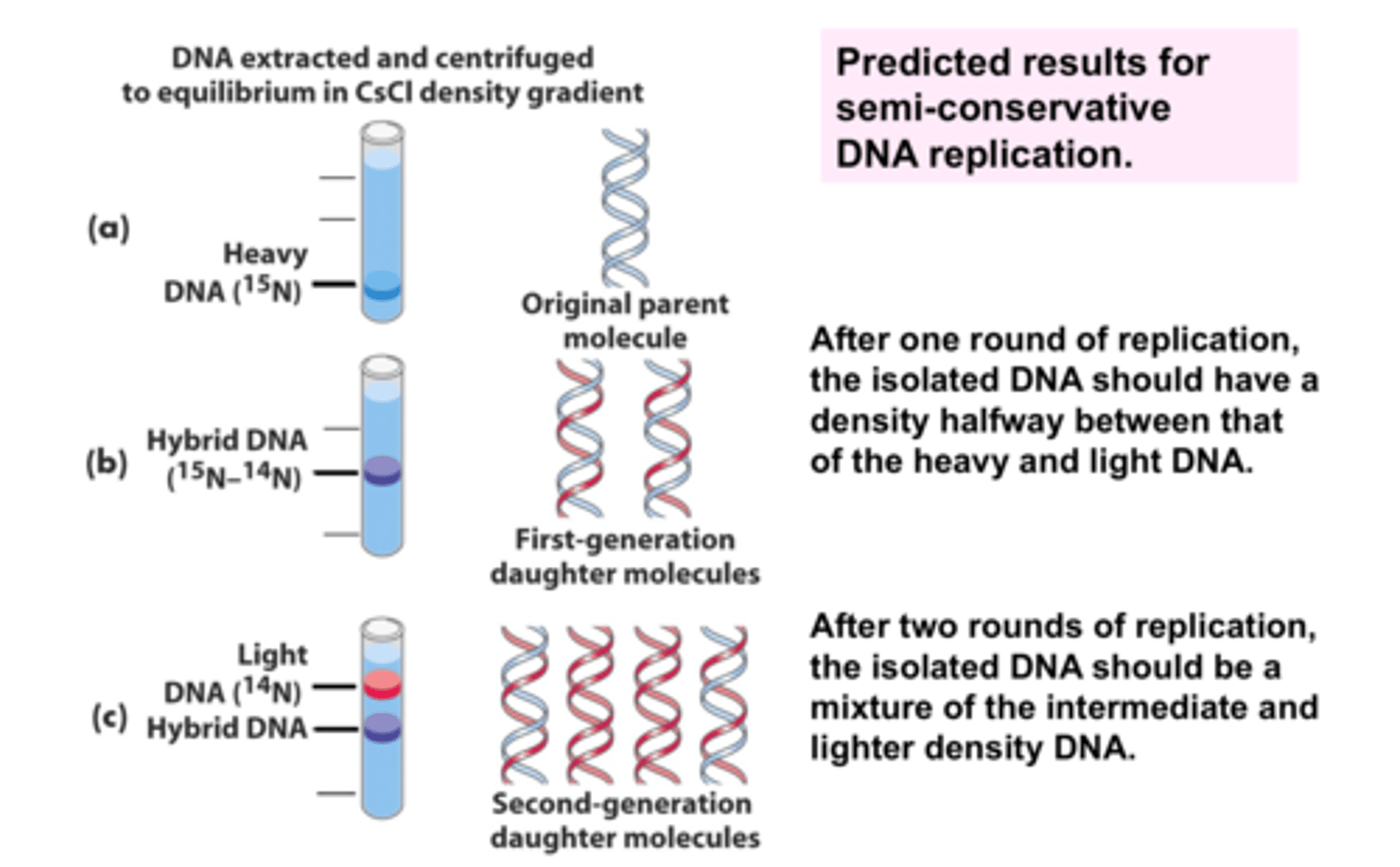
Semi-conservative - context of DNA replication
One strand of the original parental DNA
One new DNA strand
Meselson-Stahl Experiment
Used isotope of nitrogen to change the weight of DNA N15 & N14, demonstrated that the semi-conservative model is the best description of replication.
FIX
Point mutation
A point mutation is a change in a single nucleotide (base)
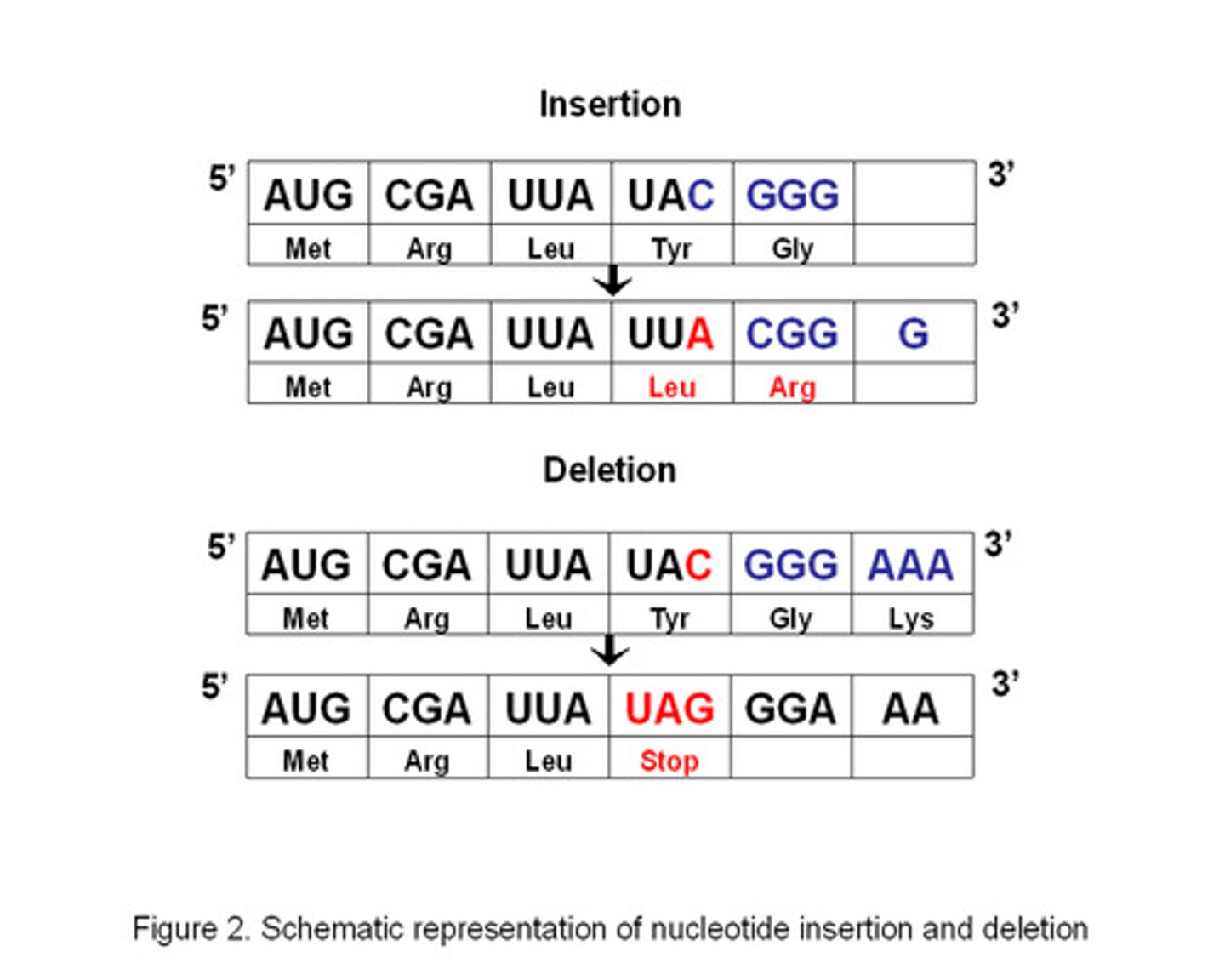
Types of frameshift mutations
Deletion & Insertion
- very serious causing a different AA sequence to be produced
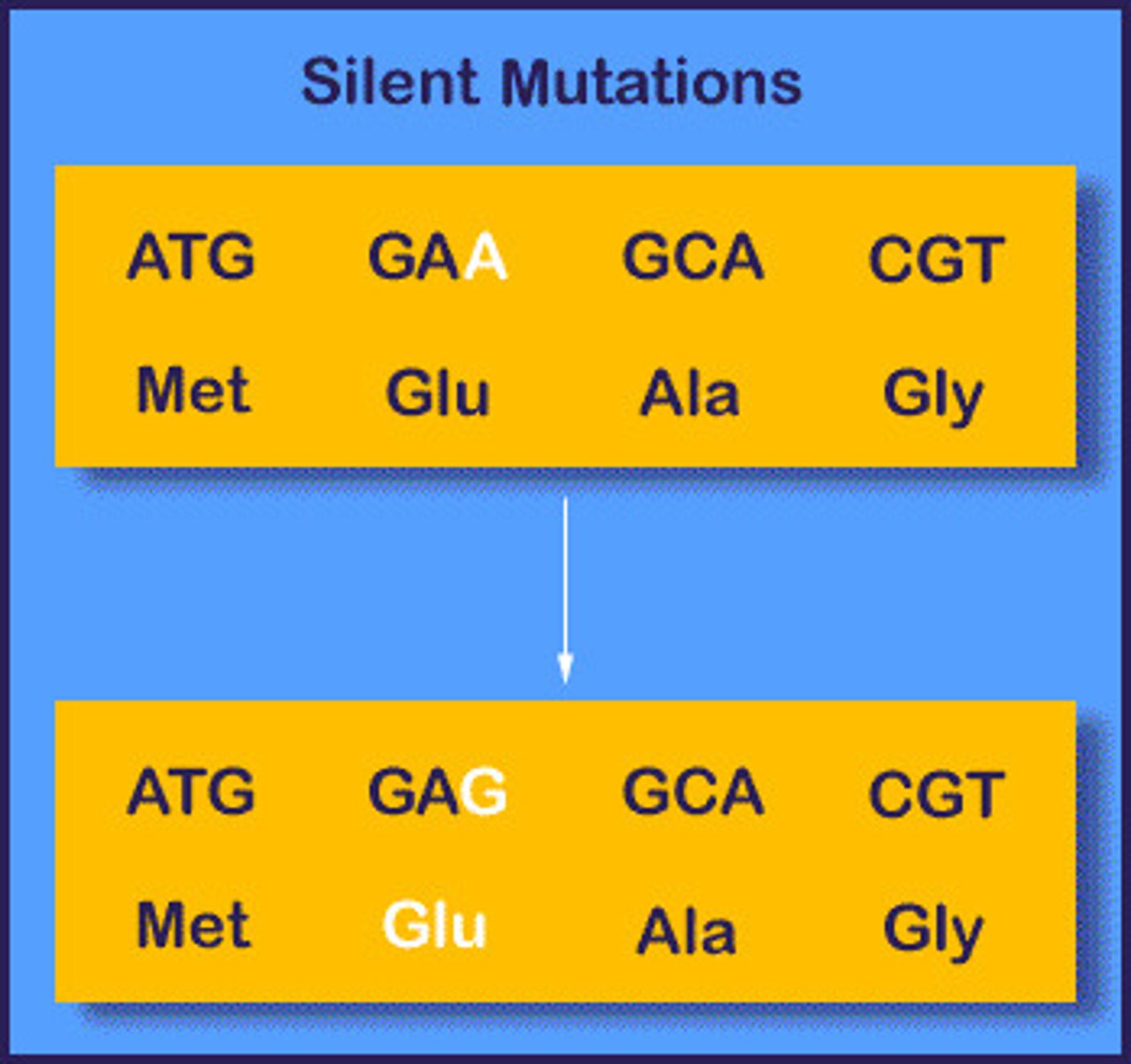
Type of silent mutation
Substitution
- less serious due to degenerate code
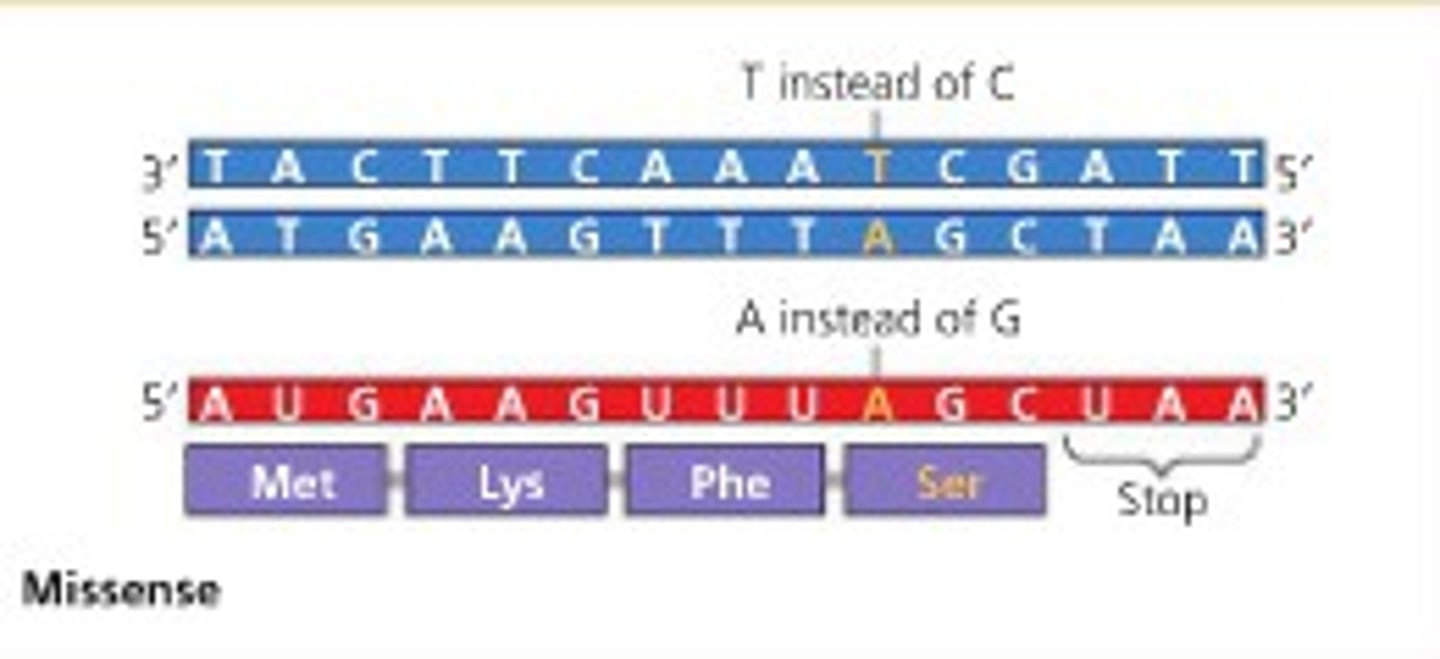
Type of missense mutations
Substitution & Inversion
- changes AA sequence
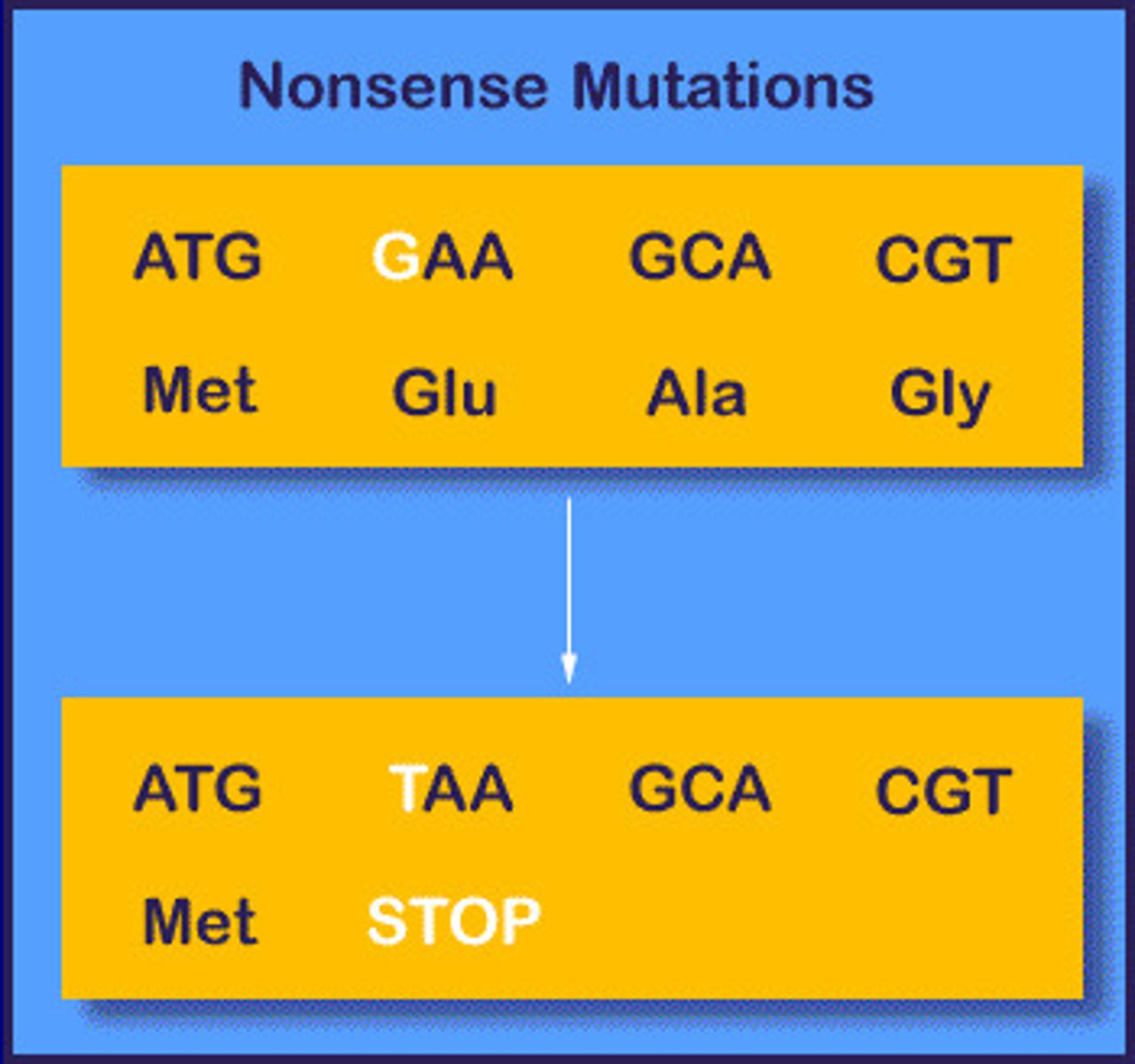
Type of nonsense mutation
Substitution
- stop codon produces a truncated protein
Mutations in CFTR gene
Almost 1400 different mutations
In 70% of cases ΔF508 mutation
mutation ΔF508 is a deletion of three nucleotides, its a missense (not frameshift because 3 codons are removed not just one) causing the AA sequence change
Gene
A sequence of bases on a DNA molecule that codes for a sequence of amino acids in a polypeptide chain
Allele
A variation of a gene
Genotype
The alleles present
Phenotype
The characteristics resulting form expression of the genotype
Recessive
An allele that is only present in the absence of a dominant allele / when homozygous
Dominant
An allele that is always expressed in the phenotype
Incomplete dominance
When the trait from a dominant allele is not completely expressed over the trait produced by the recessive allele
Homozygote
Having identical alleles on homologous chromosomes
Heterozygote
Having different alleles on homologous chromosomes v
Genetic testing (carrier testing)
Tested to see if it contains the most common mutation for a particular genetic disease
Samples of cheek cells or WBC tested
- 80% to 85% reliable
Preimplantation Genetic Diagnosis (PGD)
Fertilisation by IVF
A cell is removed from the resulting embryos at the 8-16 cell stage
The DNA is analysed
Prenatal screening - amniocentesis
During pregnancy (15-18 weeks or later)
Needle used to remove foetal cells from amniotic fluid
DNA is analysed
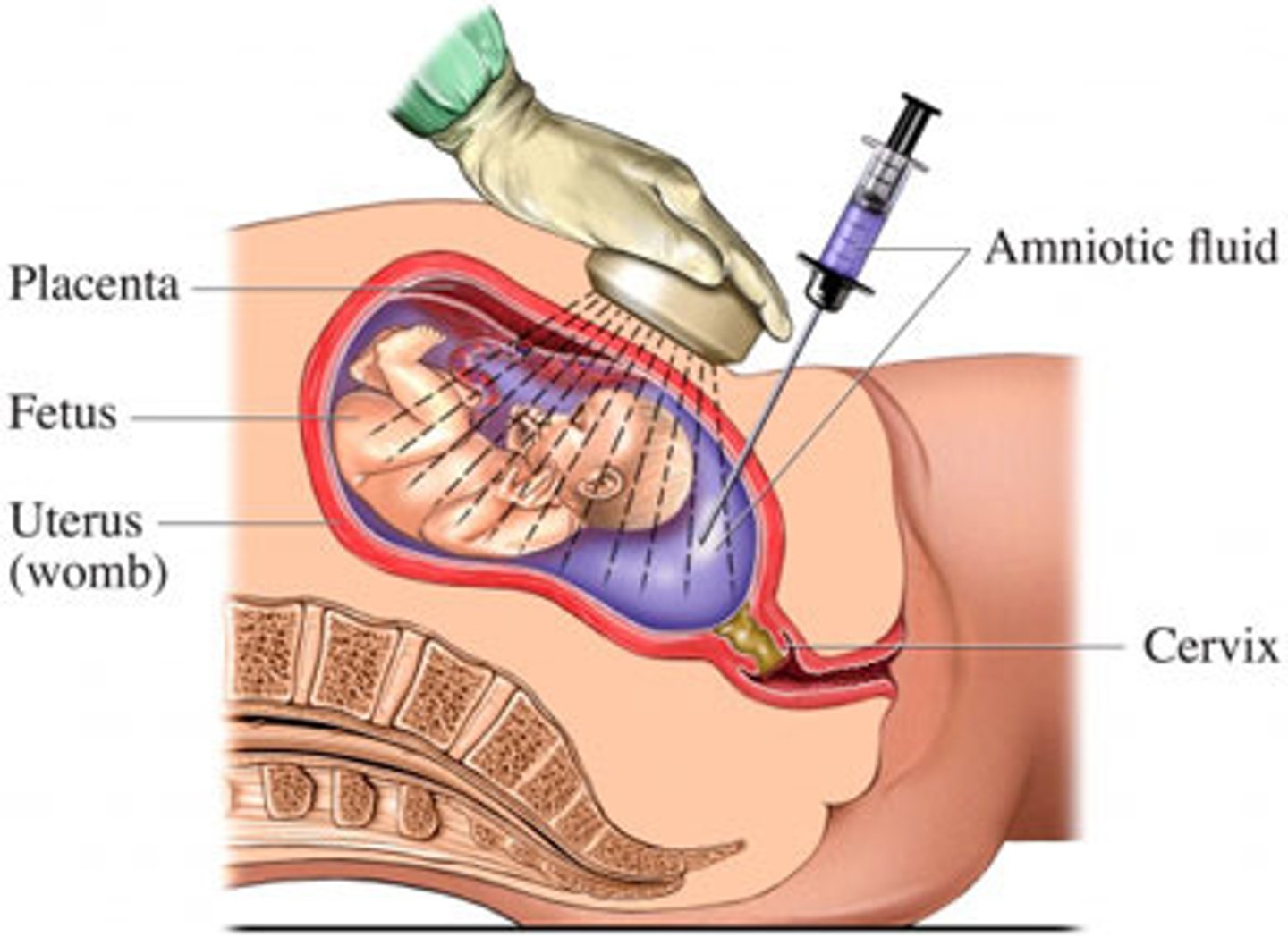
Prenatal screening - amniocentesis
(ADV vs DIS)
ADV:
1% risk of miscarriage
Results available in 3 days (NHS)
DIS
Only carried out later in pregnancy
Risk of a false positive upon which decisions including abortions could be made
Prenatal screening - chorionic villi sampling
During pregnancy from 10 weeks onwards (NHS 2019)
Suction tube used to remove foetal cells from chorion
DNA is analysed