atomic physics
1/35
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
36 Terms
isotope
an atom with the same number of protons but different amount of neutrons
ion
charged particles with different numbers of protons and electrons, creating positive or negative charge (loss of e = p, gain of e = n)
background radiation
mostly natural radiation that is always present in the environment

what is the name of the number circled?
mass number/atomic mass
what is the atomic mass/mass number?
the amount of protons and neutrons in an atom
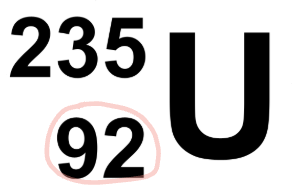
what is the name of the number circled?
the atomic number
what is the atomic number?
the atomic number is the amount of protons (and electrons) in an atom
name two types of man-made background radiation
any two from -
medical usage
fallout from nuclear weapons tests
nuclear power discharges
non-medical industry usage (eg nuclear power plant emissions)
name two types of natural background radiation
any two from -
radon in atmosphere
grounds and buildings
food and drink
cosmic rays
name two ways to keep safe whilst handling radioactive material
any two from -
wearing a special badge which shows amount of radioactive exposure
using gloves and tongs
direct sources in safe direction
put away sources in lead-lined box (when not in use)
what device is used to count the amount of radioactive particles present?
Geiger-Muller tube (G-M)
what is the mass and charge of an alpha particle
mass - 4
charge +2
what is the speed and range of an alpha particle
speed - 0.1 x speed of light
range - ~6cm
what is the mass and charge of a beta particle
mass - 1/2000
charge - -1
what is the speed and range of a beta particle
speed - 0.9 x speed of light
range - ~50cm
what is the mass and charge of a gamma ray
it has none
what is the speed and range of a gamma ray
speed - speed of light
range - almost infinite
why is a gamma ray different to an alpha and beta particle
a gamma ray is an electromagnetic wave (from the nucleus), whilst alpha and beta are particles
an alpha particle is the same as a..
helium nucleus
list the three types of radiation from most to least ionising
alpha → beta → gamma
list the three types of radiation from most penetrating to least penetrating
gamma →beta→alpha
a beta particle is the same as an..
electron
count-rate
number of decays recorded each second by a detector (eg G-M)
activity
the rate at which a source of unstable nuclei decays
measured in Becquerels (Bq)
how is alpha represented in a nuclear equation?
how is beta represented in a nuclear equation?
how is gamma represented in a nuclear equation?
radioactive decay is..
random
half-life
the time it takes for the number of nuclei of a (radioactive) isotope in a sample to halve
OR
the time it takes for the count rate (or activity) from a sample containing the isotope to halve
the shorter the half life..
the faster the decay
A radioactive isotope has a half life of 45 seconds.
If I initially have 480 particles, how long will it take for the sample to
decay to 30 particles?
30/480 = 1/16 = 1/24
45 × 4 = 180s