Exam 2 - Non-insulin Therapies, Hospital Management, Adherence
1/175
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
176 Terms
functions of GLUTs
membrane proteins containing 12 helices that transport glucose either outside OR inside of the cell; glucose binds and causes a conformational change that releases glucose to the other side of the membrane
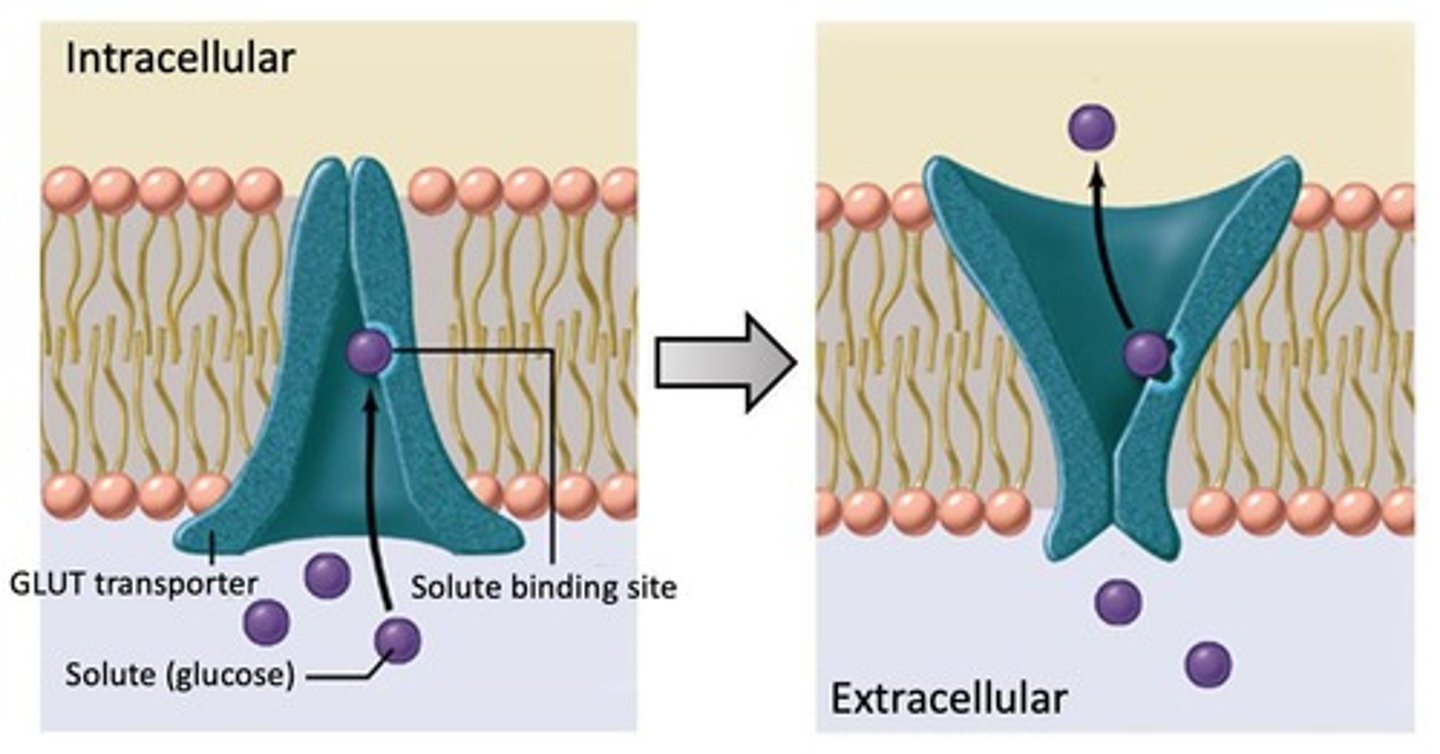
there are ____ known GLUT isoforms
14
GLUT family is divided into ___ subclasses
3
GLUT-1 function
responsible for the basal glucose uptake required to sustain respiration in ALL cells
- increased expression when REDUCED glucose
- decreased expression when INCREASED glucose
where is GLUT-1 expressed?
mostly in erythrocytes but also in barrier tissues such as the blood-brain barrier
GLUT-2 function
transfer of glucose between the liver and the blood; also has a role in renal glucose reabsorption
- increased expression during the FED state
where is GLUT-2 expressed?
liver, pancreatic beta cells, small intestine, renal tubular cells
GLUT-2 has a high capacity for _______
glucose!...but low affinity functions as a part of the glucose sensor in the pancreatic beta cells
GLUT-3 function
transports glucose into neurons (BBB and astrocytes)
where is GLUT-3 expressed?
mostly in the brain
GLUT-4 function
insulin-regulated glucose transporter; during low insulin, GLUT-4 is sequestered in intracellular vesicles of muscle and fat, but in response to insulin signaling, the vesicles are transported to the membrane and GLUT-4 transporters become available for transporting glucose INTO the cell (increased glucose absorption); glucose is then rapidly stored as glycogen
where is GLUT-4 expressed?
mostly in adipose tissues and striated muscle
GLUT-5 function
fructose transporter
where is GLUT-5 expressed?
apical border of enterocytes, skeletal muscle, testis, kidneys, adipocytes, brain
HbA1c and glucose equilibrium
glucose is in equilibrium with a hexose ring-close and ring-open form, which possesses an aldehyde at C6 that undergoes a glycation reaction with hemoglobin in a red blood cell with the C1 on glucose reacting with the N-terminal valine on hemoglobin
- gives a "history" of serum glucose concentrations over 8-12 weeks (2-3 months)
- normal is 4-6%,
- <7% is good maintenance in diabetics
diagnostic criteria for diabetes (4)
- FPG ≥126 mg/dL
- 2-hours post 75g-oGTT ≥200 mg/dL
- random PG of ≥200 mg/dL
- A1c ≥6.5%
which therapies cause weight GAIN?
- insulin
- sulfonylureas
- meglitinides
- TZDs
which therapies cause weight LOSS?
- GLP-1 RAs
- SGLT2 inhibitors
which therapies are weight NEUTRAL?
- metformin
- DPP-4 inhibitors
- colesevelam
- α-glucosidase inhibitors
- pramlintide
- bromocriptine
therapies that act in the small intestine?
- metformin
- DPP-4 inhibitors
- α-glucosidase inhibitors
therapies that act in the pancreas?
- GLP-1 agonists
- sulfonylureas
- meglitinides
therapies that act in adipose tissue?
TZDs
therapies that act in the kidneys?
SGLT2 inhibitors
therapies that act in the liver?
metformin
name the 3 sulfonylureas
glipizide, glyburide, glimepiride
name the biguanide
metformin
name the 2 glycosidase inhibitors
acarbose, miglitol
name the 2 meglitinides
natelinide, repaglinide
name the 2 TZDs
pioglitazone, rosiglitazone
MoA of sulfonylureas
increase glucose uptake in muscle by stimulating insulin release from pancreatic beta cells by binding to the SUR1 channel to artificially inhibit KATP channels (keeping them closed)
concerns with sulfonylureas
- will not work in type 1 diabetics or type 2 diabetics whose beta cells no longer produce insulin
- weight gain
- hypoglycemia
- DDIs since they bind to albumin
DDIs with sulfonylureas
- NSAIDs (salicylates)
- sulfonamides (chloramphenicol)
- coumadin
- probenecid
- thiazides
- phothiazides
- thyroid products
- oral contraceptives
- phenytoin
sulfonylurea structure
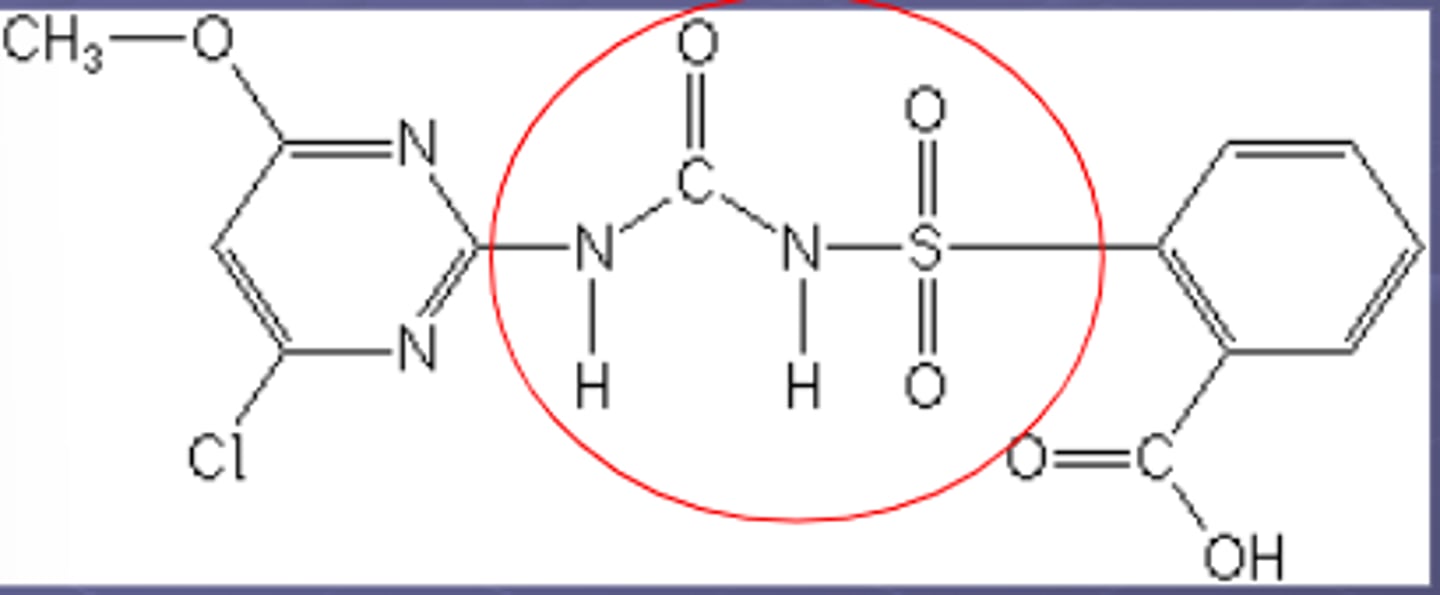
2nd generation sulfonylureas
glipizide and glyburide
MoA of meglitinides
bind to SUR1-KATP channels at sites that differ from sulfonylureas (but otherwise share the same mechanism) and are metabolized by CYP450/2C8/3A4
solubility of metformin
very soluble
permeability of metformin
poor
charge of metformin at physiological pH
cation (ionized form)
LogP of metformin
negative (more molecules in the water phase)
elimination/half-life of metformin
fast elimination, short half-life
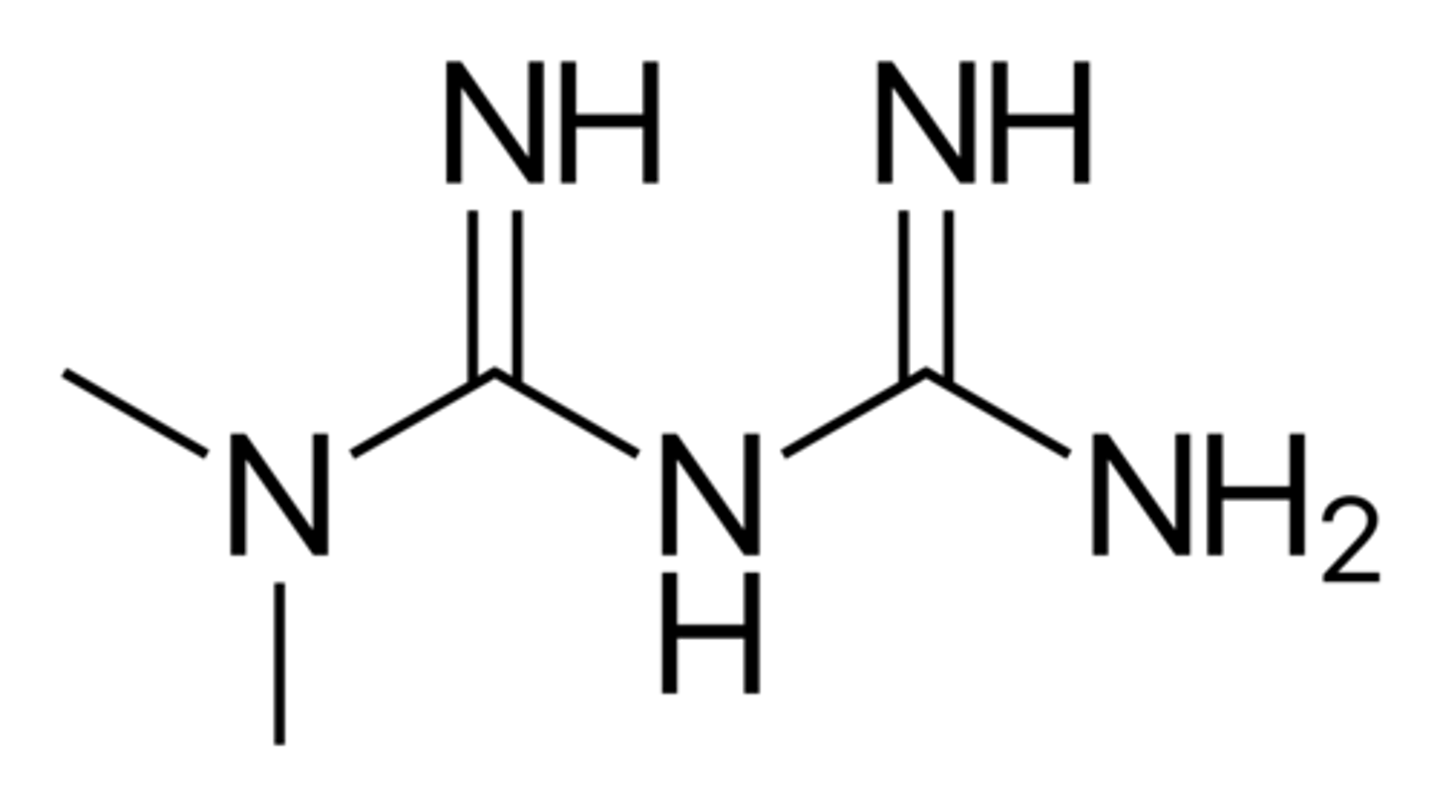
metformin structure
solubility of glipizide
poor
permeability of glipizide
medium
charge of glipizide at physiological pH
neutral (unionized)
metformin: basic/acidic/neutral?
strong base
glipizide: basic/acidic/neutral?
slightly weak acid
LogP of glipizide
positive (more molecules in the oily phase)
glipizide structure
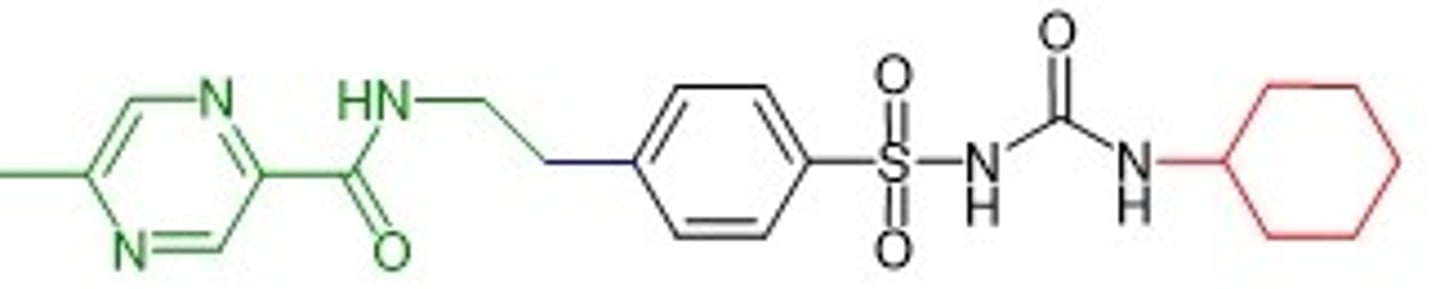
elimination/half-life of glipizide
higher protein binding so longer half-life
solubility of empagliflozin
high (despite the molecule itself being insoluble, it is high due to the dose being so small)
permeability of empagliflozin
poor
empagliflozin: basic/acidic/neutral?
neutral
charge of empagliflozin at physiological pH
neutral
LogP of empagliflozin
positive (more molecules in the oily phase)
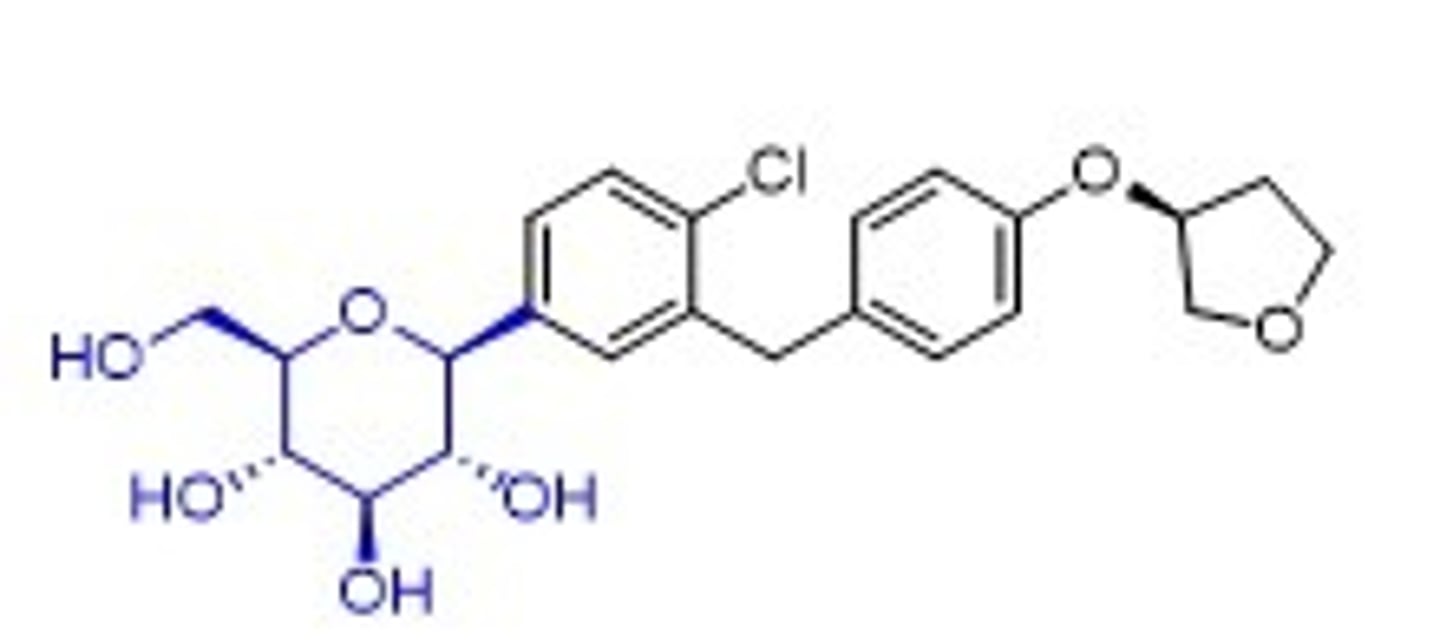
empagliflozin structure
gastro-retention
delivery strategy used to retain drugs in the stomach and let them slowly move into the upper GI tract (avoids gastric emptying)
5 ways to accomplish gastro-retention in formulation of tablets
1) high-density systems that withstand peristalsis
2) swelling/expansion forms to prevent passage
3) adhesion to the stomach lining
4) superporous hydrogels
5) floating drug delivery
Glumetza formulation
metformin HCl extended release that is formulated with hypromellose (rate-controlling polymer layer) and polyethylene oxide (swelling agent)
- keeps the dosage form within the stomach
- slows release through the hydrophilic polymer

osmotic pump formulation
tablet formulation that allows for an extended-release profile
- core containing the osmotic agent
- rigid, semi-permeable membrane surrounding the core
- delivery orifice (laser-drilled) to release the drug
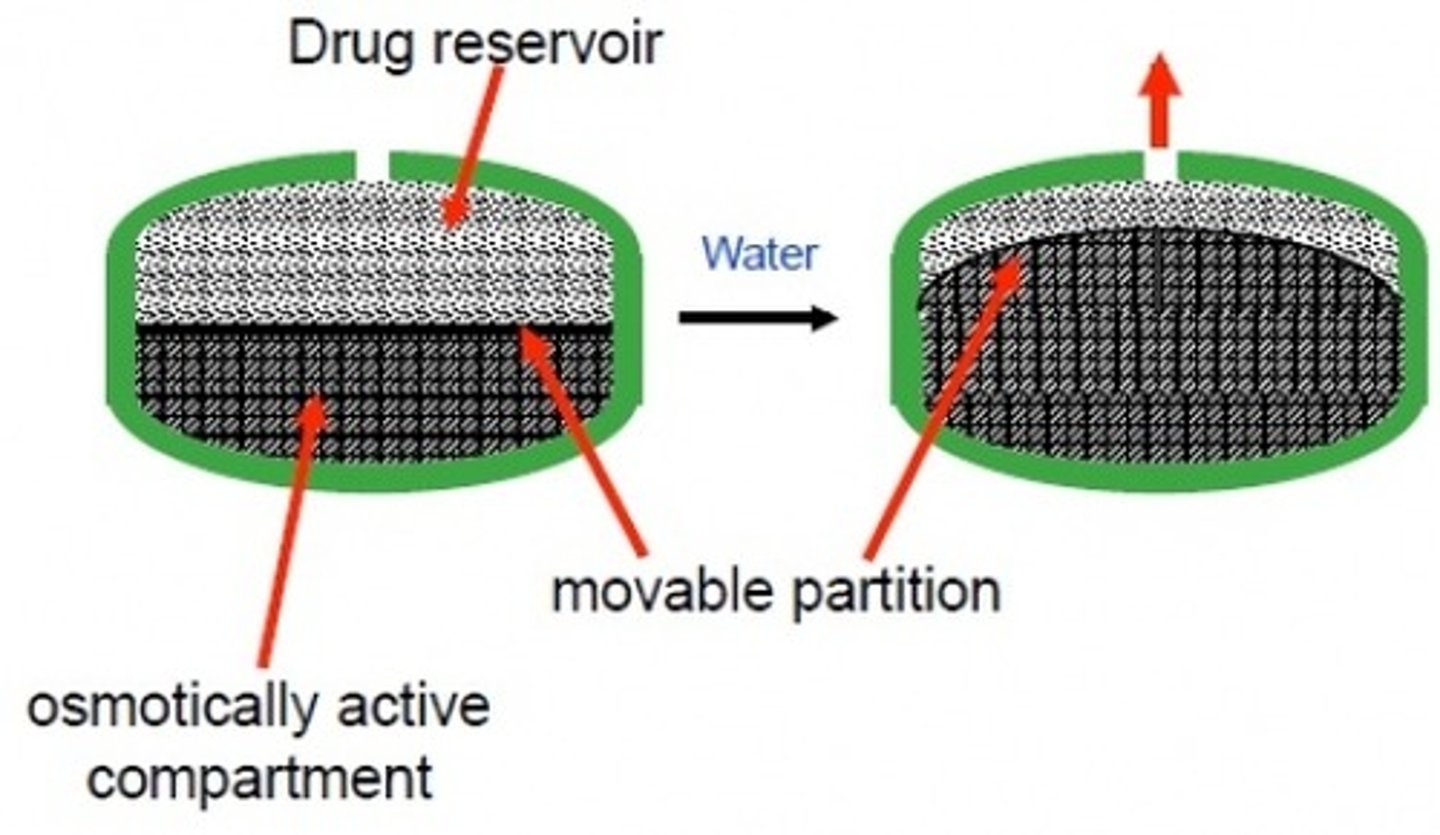
3 key principles that define drug release from an osmotic tablet
1) water influx
2) interior solution formation
3) drug release
water influx equation
Jw = A x P x (Δπ/h)
Jw = flux of water
A = tablet geometry
P = permeability
Δπ = osmotic gradient
h = thickness
Fortamet formulation
ER metformin formulated as a single-composition osmotic technology (SCOT)
SCOT formulation
ER tablet that contains an osmotically active core formulation surrounded by semipermeable membrane -> water diffuses past the membrane to dissolve the drug -> drug can't move past the membrane = osmotic pressure
push-pull osmotic technology
two compartment ER model that contains one compartment of drug and one compartment of osmotic agent; the osmotic agent pushes the drug outside of the membrane
requirements for a semipermeable membrane
- sufficient strength
- biocompatible
- rigid and non-swelling
common membrane excipients
ethyl cellulose, cellulose acetate
are Fortamet and Glumetza interchangeable?
NO
(although they are both ER formulations of metformin, they have different release mechanisms leading to different PK responses)
formulation of glipizide
low solubility, so requires a two-compartment push-pull osmotic delivery system requiring a separate osmotic agent and a physical moveable partition
- lower chamber swells and pushes the dissolved drug through the laser-drilled orifice at a controlled rate
formulation design of semaglutide
purpose is to keep the peptide stable and in solution, so is formulated with a disodium phosphate dihydrate buffer to maintain pH, phenol as an antioxidant, and propylene glycol as a co-solvent with water
primary structure of semaglutide at pH 7.4
net charge is -4; everything will be ionized
- 4 acidic residues
- 3 basic residues
- 2 additional carboxylic acids on the Lys26
formulation of rybelsus
very low bioavailability, so is formulated with SNAC to enhance GI permeation
SNAC
salcaprozate sodium
- GI permeation enhancer used in rybelsus
- critical for making an oral peptide work
mechanism of SNAC
mostly unclear mechanism
- absorption only in the stomach
- surrounds semaglutide and neutralizes gastric fluids and inactivates pepsin
- boosts local solubility to drive transcellular uptake
macrovascular complications of diabetes
ASCVD and heart failure
microvascular complications of diabetes
nephropathy/CKD, neuropathy, retinopathy
"high risk" of developing ASCVD
age ≥55 with 2+ risk factors:
- obesity
- HTN
- smoking
- dyslipidemia
- albuminuria
first-line diabetes treatment for ASCVD and high ASCVD risk
SGLT2i and/or GLP-1 RA
recommended SGLT2i for ASCVD + diabetes
empagliflozin
recommended GLP-1 RA for ASCVD + diabetes
semaglutide
HFrEF vs HFpEF LVEF %
HFrEF ≤40%
HFpEF ≥50%
first-line therapy for HF + diabetes
SGLT2i
GLP-1 RAs for HF + diabetes?
not guideline recommended since they do not have proven CV benefit in patients with HF... still waiting for more trial data to come out
pathophysiology of nephropathy/CKD and diabetes
hyperglycemia and insulin resistance -> increased oxidative stress, inflammation, and overactive RAAS -> glomerular HTN and renal fibrosis
nephropathy/CKD occurs in ______% of people with diabetes
20-40
when to screen diabetics for nephropathy/CKD?
at least annually...
T1D: 5 years after diagnosis
T2D: at diagnosis
CKD diagnostic criteria
eGFR <60 or albuminuria UACr ≥30
first-line therapy for CKD + diabetes
SGLT2i, semaglutide, or ACEi/ARB
when to consider nonsteroidal MRA in CKD + diabetes?
if albuminuria persists and pt has normal potassium after max tolerated ACEi/ARB
in CKD, decision to use a GLP-1 RA or SGLT2i with proven benefit should be made irrespective of...
background use of metformin or A1c
first-line therapy for MASLD/MASH + diabetes
GLP-1 RA, terzepatide, pioglitazone
MASLD
metabolic dysfunction associated steatotic liver disease
- hepatic steatosis in the presence of at least one metabolic risk factor with alcohol consumption below thresholds likely to cause liver injury
MASH
metabolic dysfunction associated steatohepatitis
- progressive, inflammatory form of MASLD
pathophysiology of neuropathy and diabetes
increased serum glucose -> insulin resistance, dyslipidemia, oxidative stress -> inflammation and cellular damage to nerve fibers
peripheral vs autonomic neuropathy
peripheral: screen with diabetic foot exam
- up to 50% may be asymptomatic
- burning, sharp pain
- cold sensation
- numbness
autonomic: screen for symptoms
- orthostatic hypotension
- resting tachycardia
- dry or cracked skin in the extremities
- constipation/diarrhea
- erectile dysfunction
when to screen diabetics for neuropathy?
at least annually...
T1D: 5 years after diagnosis
T2D: at diagnosis
pathophysiology of retinopathy and diabetes
hyperglycemia +/- uncontrolled blood pressure damages the blood vessels supplying blood to the retina of the eye
when to screen diabetics for retinopathy?
at least annually with dilated and comprehensive eye exams...
T1D: 5 years after diagnosis
T2D: at diagnosis
treatment of retinopathy
no specific pharmacotherapy recommended to treat or prevent progression, just need to adequately control glucose concentrations and blood presure
4 pillars of reducing complications in diabetes
- glycemic management
- blood pressure management
- lipid management
- agents with cardiovascular and kidney benefit
goal A1 range to reduce complications from diabetes
≤7