Need to Know
1/178
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
179 Terms
Every element has a unique atomic number representing the
number of protons
Mass Number
Number of Protons and Neutrons
Mass Number is located where on an element
in the upper left corner of its symbol
Atomic number is located where on an element
in the lower left corner of its symbol
isotope
a variant of an element, having the same number of protons but different numbers of neutrons.
Protium
the most abundant hydrogen isotope, with one proton and no neutrons.
Deuterium
a hydrogen isotope with one proton and one neutron.
Tritium
a hydrogen isotope with one proton and two neutrons, known for its radioactive properties.
Iron has how many isotopes
31
Iron has how many stable isotopes
4 stable isotopes: Fe-54, Fe-56, Fe-57, and Fe-58.
There are about ____ known naturally occurring and synthetic isotopes
3000
there are predicted to be up to ____ isotopes that could possibly exist
7000
How many isotopes are stable on Earth
250 and include only elements below Pb
Pb has the
Heaviest stable Isotopes
Smaller elements are stable with a
1:1 ratio of neutrons and protons
Large nuclei need
increasing numbers of neutrons for the isotopes to be stable
Fission
The splitting of large unstable isotopes. This is the basis for the first nuclear bombs as well as nuclear power plants
Fusion
The combining of small particles in a highly exothermic process. It occurs within the sun and is potentially the ideal source of clean nuclear power to solve the world’s energy crisis. Technological challenges have slowed progress on the development of fusion reactors
Radioactive Decay
The process by which the approximately 7000 isotopes decay to the 250 stable isotopes on Earth. There are many types of radioactivity that involve small particles like photons, protons, electrons, positrons, alpha particles, etc., to be emitted with a half-life that can be extremely fast or millions of years. The types of radioactive decay you need to be responsible for are: alpha decay, beta decay and gamma decay
Alpha particle (Notation)

Beta particle (Notation)
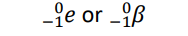
Gamma radiation (Notation)
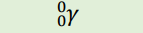
Neutron (Notation)
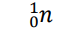
Proton (Notation)
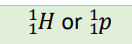
Positron (Notation)
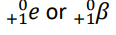
Alpha particle (Symbol)
α
Beta particle (Symbol)
β —
Gamma radiation (Symbol)
γ
Neutron (Symbol)
n
Proton (Symbol)
p
Positron (Symbol)
β +
Nuclear energies =
millions to billions of kJ/mol
Chemical bond energies =
100s to 1000s of kJ/mol
Intermolecular forces =
10s of kJ/mol
α decay
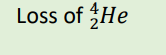
β decay

γ decay

α decay (Example)
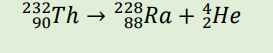
β decay (Example)
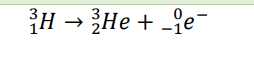
γ decay (Example)
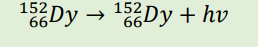
conservation of mass still applies here
the mass numbers on the left and right sides should add to the same number, and the protons on the left and right sides should add to the same number. Looking at β decay, for example: 3 + 0 = 3 (mass numbers) and 2 + (-1) = 1 (protons)
A common question is to be given the famous decay cycle
238U to 206Pb, which occurs by a 14-step process that will take billions of years to happen
A species’ half-life is
the amount of time it takes for the initial concentration of that species to be halved
0th order (half-life equation)
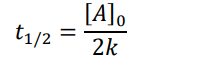
1st order (half-life equation)
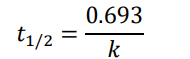
2nd order (half-life equation)
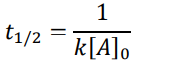
How to solve half life problems
Dividing by 2 because we’re dealing with half-life math here: 0.4 → 0.2 → 0.1 → 0.05 We divided by 2 three times to get from the start to the end. This means in 6 seconds, we used three half-lives. 6 seconds 3 half − lives = 2 𝑠𝑒𝑐𝑜𝑛𝑑𝑠 ℎ𝑎𝑙𝑓 − 𝑙𝑖𝑓𝑒 So, one half-life is 2 seconds.
Differential Rate Law: Rate Expression
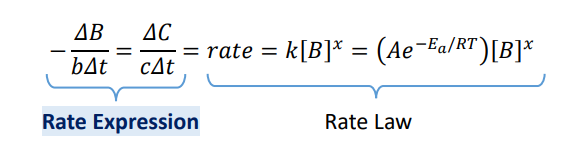
0th order (Rate Expression)
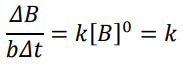
1st order (Rate Expression)
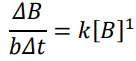
2nd order (Rate Expression)
0th order (Units)
Msec-1
1st order (Units)
sec-1
2nd order (Units)
M-1sec-1
Zeroth Order (Integrated Rate Law)
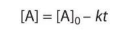
First Order (Integrated Rate Law)
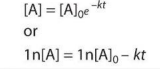
Second Order (Integrated Rate Law)
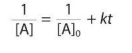
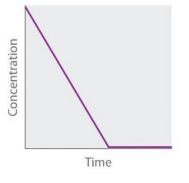
Zeroth Order (Concentration vs time graph)
First Order (Concentration vs time graph)
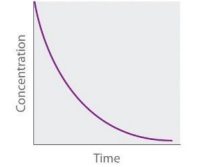
Second Order (Concentration vs time graph)
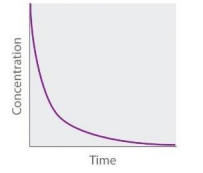
Zeroth Order (Straight Line plot to determine rate law constant)
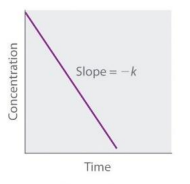
First Order (Straight Line plot to determine rate law constant)
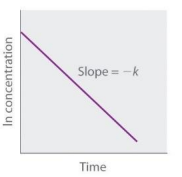
Second Order (Straight Line plot to determine rate law constant)
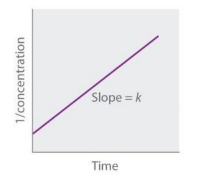
Method of Initial Rates (You got this down)
Integrated Rate Laws
Where [A]0 is the initial concentration, k is the rate constant, [A] is the concentration after some amount of time, t, and a is the coefficient in the reaction. If not explicitly provided, assume a = 1
Recognize that a straight-line formula follows the famous
y = mx + b equation, where y is the dependent variable (y-axis), m is the slope, x is the dependent variable (x-axis), and b is the y-intercept
Arrhenius equation
Where k is the rate constant, Ea is the activation energy, R is the gas constant, A is a constant called the pre-exponential factor, and T is the temperature
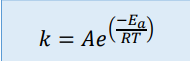
The pre-exponential factor is specific to the reaction
it represents the frequency of collisions between reactant molecules with the correct orientation for a reaction to occur
This equation highlights the relationship between the rate constant and temperature
as temperature increases, k increases. Logically this is because as the temperature gets higher, molecules have more energy to overcome the activation barrier to the reaction
Using two sets of data points, the equation can be expressed as
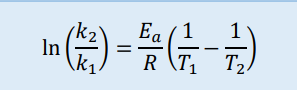
Arrhenius Equation
• This equation is similar to the van’t Hoff and ClausiusClapeyron equations.
• Activation energy (Ea) is the barrier to a reaction and is always
positive, on the order of about 5-500 kJ/mol.
• Thus, increasing T means k increases.
• Concentration vs time at two temperatures yields data that
can be plugged in to the equation to solve for Ea.
Combined Arrhenius Calculation
Collision theory is based on the
kinetic molecular theory of gases
This means 𝐾𝐸 = 1/2 𝑘𝑇 = 1/2 𝑚𝑣2
Collision theory says
collision must occur for the reaction to happen. (Note this does not explain first order decay.)
For the reaction to occur
the collision must be effective.
What determines an effective collision
This requires:
1. Sufficient energy to overcome the activation barrier (high enough temperature)
2. Correct orientation of collision
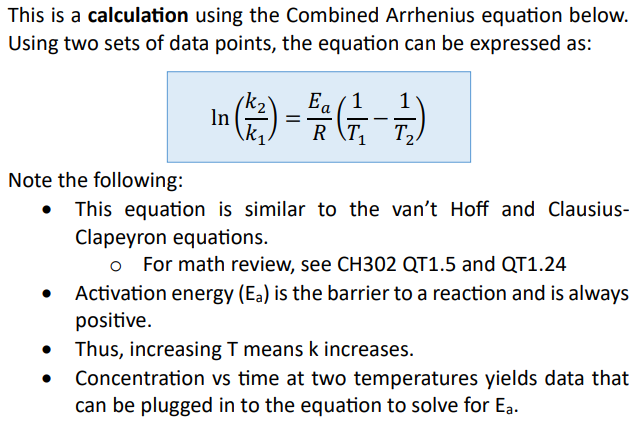
Transition states are
most easily depicted on reaction profiles of energy vs. time (reaction progress)
Transition State Theory is based on
energy, not collisions
In this profile (Transition State Theory)
an energy hill called the “activation barrier” rises positively above the thermodynamic profile
The barrier in transition State Theory is the reason why
spontaneous processes don’t happen without a source of energy (like why hydrogen balloons don’t explode unless they are touched by a flame)
The top of the activation barrier is the
“transition state” or “activated complex”. It is a theoretical structure. It is NOT an intermediate in the reaction
At the top of the energy hill
a transition state is equally likely to fall to the left or right
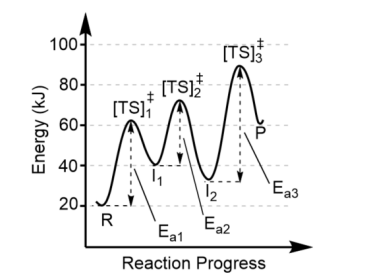
Reactants (R) are the
on the left
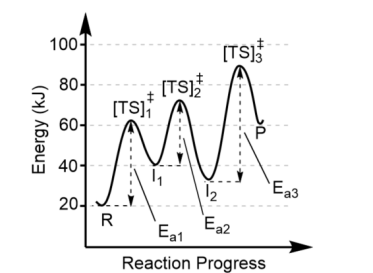
Products (P) are the
valley on the right
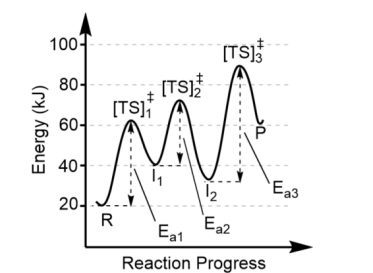
Transition states are found at
the top of each energy hill, [TS]‡
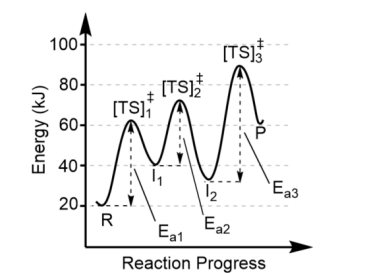
There is one transition state for
each step in the mechanism, thus this reaction has 3 steps (3 “hills” = 3 transition states)
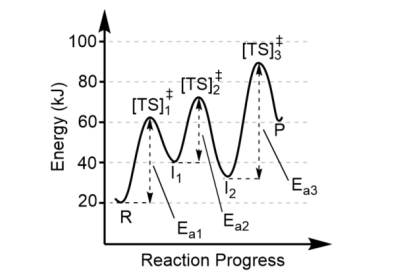
Each valley between reactants and products represent
the intermediates (I)
There can be more than one intermediate made, so the minimum number of intermediates is one less than the number of steps in the mechanism. Here, there must be at least two intermediates
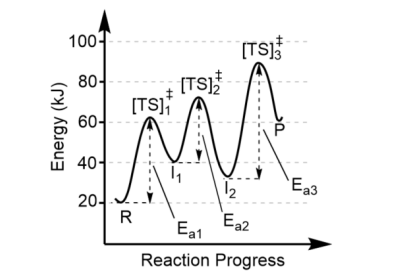
The largest activation barrier is associated with
the slow step, or the rate determining step, in the reaction. Here, that is Step 3 because Ea3 is the largest.
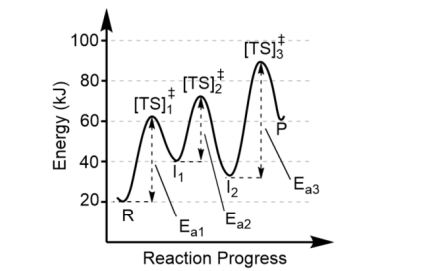
Note the forward reaction is
endothermic (Product energy – Reactant energy = positive
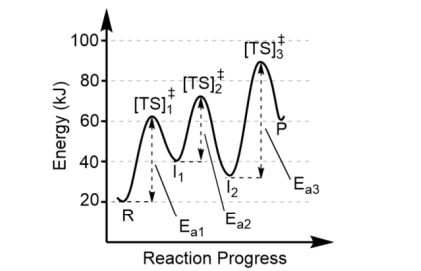
The reverse reaction is
exothermic
A catalyst typically shows up
as a reactant first and is then reproduced later in the reaction
an intermediate is
produced first then used up in a later step
To find the rate law given the slow step
first ignore everything from the products of that step and beyond. Then, cross out any remaining species that show up on both sides of the reaction arrow, and what’s left is the rate law
Rate Determining Step

Elementary Reactions


Given the reaction mechanism above, which species is a catalyst?
a) ClO
b) ClO2
c) NO2
d) None of these
e) N2O
D
![<p>Given the reaction mechanism above, what is the rate law?</p><p>a) Rate = k[NO2Cl]2[NO2]</p><p>b) Rate = k[NO2Cl]</p><p>c) Rate = k[NO2Cl]2[NO2]-1</p><p>d) Rate = k[N2O][NO2]-1</p><p>e) Rate = k[NO2Cl][NO2][NO2]-1</p>](https://knowt-user-attachments.s3.amazonaws.com/b111ab32-aadc-4205-a665-ad84c80f943e.png)
Given the reaction mechanism above, what is the rate law?
a) Rate = k[NO2Cl]2[NO2]
b) Rate = k[NO2Cl]
c) Rate = k[NO2Cl]2[NO2]-1
d) Rate = k[N2O][NO2]-1
e) Rate = k[NO2Cl][NO2][NO2]-1
C
Ozone Layer Decomposition (Reactants)
2 O3 (g)
Ozone Layer Decomposition (Products)
3 O2 (g)
Ozone Layer Decomposition
Homogeneous chlorine radical, Cl•, catalyst