Orgo Quizzes 1-12
1/296
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
297 Terms
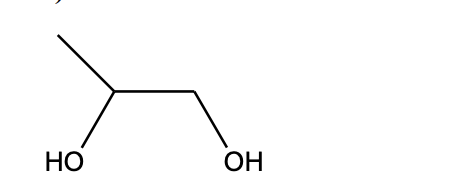
IUPAC Name
1,2-propanediol
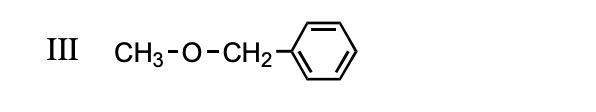
Structure of Benzyl Methyl Ether
Which of the compounds listed below would you expect to have the highest boiling point? (They all have approximately the same molecular weight.)
CH3CH2CH2CH2OH
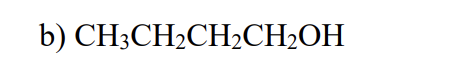
Which compound would have the lowest solubility in water?
Pentane
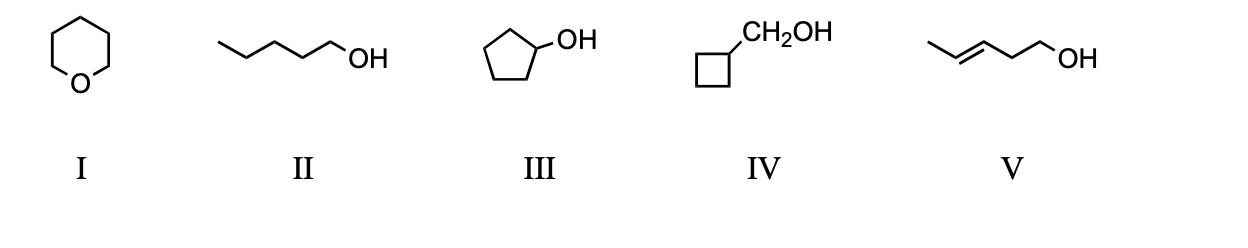
Which compound would have the lowest boiling point?
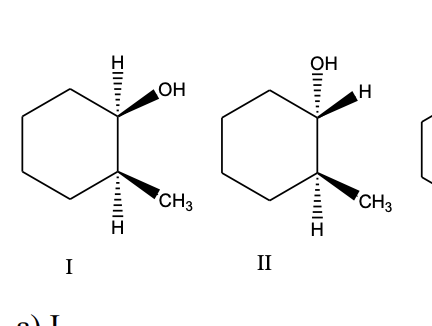
a) I
Which of the following statements is NOT true of ethers?
Ethers are generally unreactive molecules toward reagents other than strong acids.
b) Ethers generally have lower boiling points than alcohols of a corresponding molecular weight.
c) Ethers generally have much higher water solubilities than alcohols with a corresponding molecular weight.
d) Ethers can generally be cleaved by heating them with strong acids. e) Ethers form peroxides when allowed to stand in the presence of oxygen
C) Ethers generally have much higher water solubilities than alcohols with a corresponding molecular weight.
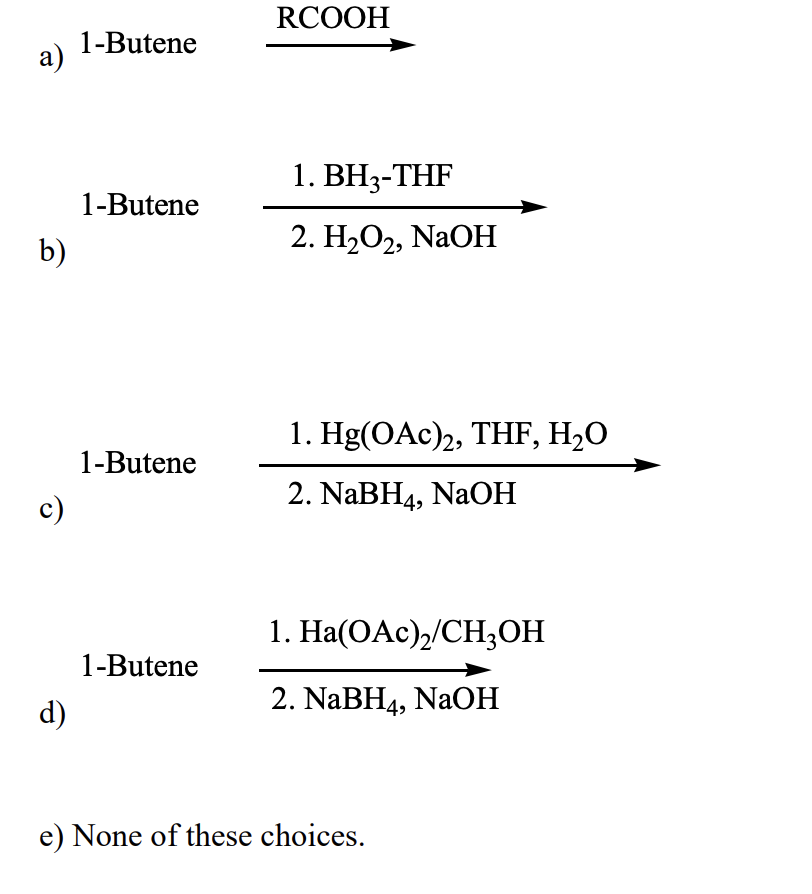
Which of the following would be a reasonable synthesis of 2-butanol?
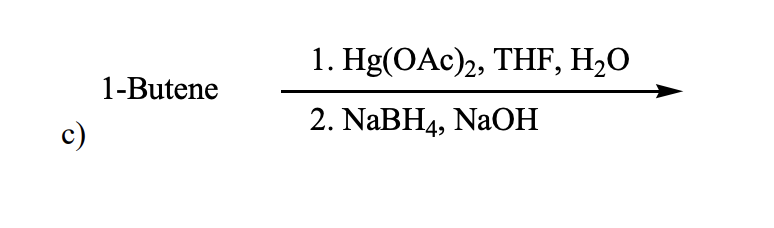
Which would be the best method for converting 3,3-dimethyl-1-pentene into 3,3-dimethyl-2-pentanol?
a) H3O + , heat
b) BH3:THF; then H2O2, OH–
c) concd. H2SO4; then H2O, heat
d) Hg(OAc)2/THF-H2O; then NaBH4,OH–
e) HBr; then NaOH/H2O
D) Hg(OAc)2/THF-H2O; then NaBH4,OH–
Assuming the mechanistic pathway for each of the following reactions is predominately SN1, which of these alkyl halide syntheses is predicted to occur at the greatest rate?
a) CH3CH2CH2CH2OH + HI →
b) (CH3)2CHCH2OH + HBr →
c) CH3CHOHCH2CH3 + HCl →
d) CH3CHOHCH2CH3 + HBr →
e) (CH3)3COH + HI →
e) (CH3)3COH + HI →
10) Which statement is true concerning the formation of alcohols by the hydroboration-oxidation sequence?
a) Overall, the process results in syn addition and anti-Markovnikov orientation.
b) Overall, the process results in anti addition and anti-Markovnikov orientation.
c) Overall, the process results in syn addition and Markovnikov orientation.
d) Overall, the process results in anti addition and Markovnikov orientation.
e) The stereochemistry and orientation are unpredictable.
a) Overall, the process results in syn addition and anti-Markovnikov orientation.
Which of the following could be used to synthesize 1-bromobutane efficiently?
a) CH3CH2CH=CH2 + HBr →
b) CH3CH2CH2CH2OH + PBr3 →
c) CH3CH2CH2(OH)CH3 + HBr →
d) CH3CH2CH2CH2OH + Br2 →
e) None of these choices.
b) CH3CH2CH2CH2OH + PBr3 →
What is the product of the reaction of propyl alcohol with (CH3)3SiCl in the presence of a tertiary amine?
a) CH3CH2CH2Si(CH3)3
b) (CH3)2CHSi(CH3)3
c) CH3CH2CH2OSi(CH3)3
d) (CH3)2CHOSi(CH3)3
e) (CH3CH2CH2)3SiOH
c) CH3CH2CH2OSi(CH3)3
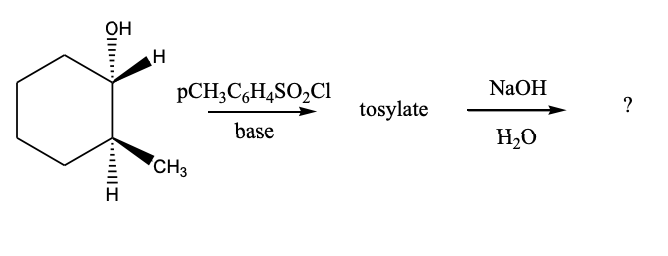
What would be the major product of the following reaction sequence?
Which is the best way to prepare 3-methoxypentane via the Williamson method?
a) CH3OH + CH3CH2CHOHCH2CH3 + H2SO4, 140 °C
b) CH3OH + (CH3)2CHCH2CH2OH + H2SO4, 140 °C
c) CH3ONa + (CH3CH2)2CHBr
d) CH3I + (CH3CH2)2CHONa
e) CH3I + (CH3)2CHCH2CH2ONa
d) CH3I + (CH3CH2)2CHONa
When 3-methyl-2-pentene is treated with mercuric acetate, Hg(O2CCH3)2, in a THF-t-butyl alcohol mixture and the resulting product reacted with NaBH4 in basic solution, the principal product formed is which of these?
a) 3-methyl-3-pentanol
b) 3-t-butoxy-3-methylpentane
c) 3-methyl-2-pentanol
d) 2-t-butoxy-3-methylpentane
e) 1-t-butoxy-3-methylpentane
3-t-butoxy-3-methylpentane
16) Which of these ethers is most resistant to peroxide formation on exposure to atmospheric oxygen?
a) CH3OCH2CH3
b) CH3CH2OCH2CH3
c) (CH3)2CHOCH(CH3)2
d) (CH3)2CHOCH2CH3
e) CH3OC(CH3)3
e) CH3OC(CH3)3
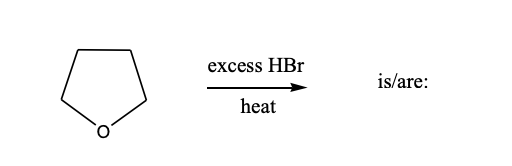
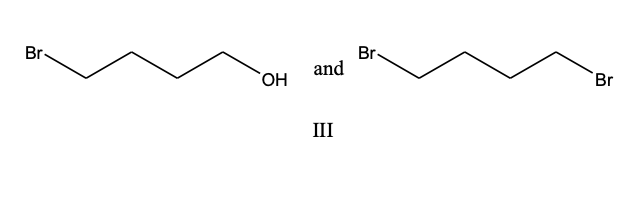
The product(s) of the following reaction
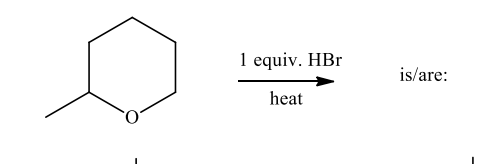
The product(s) of the following reaction
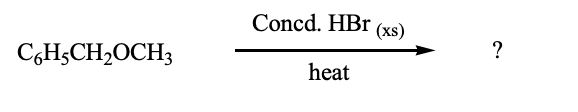
What would be the major product(s) of the following reaction (Concd = Concentrated; xs = excess)?
b) C6H5CH2Br + CH3Br
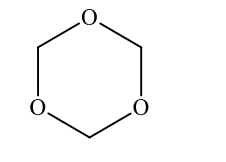
A correct IUPAC name for the following compound is?
6-crown-3
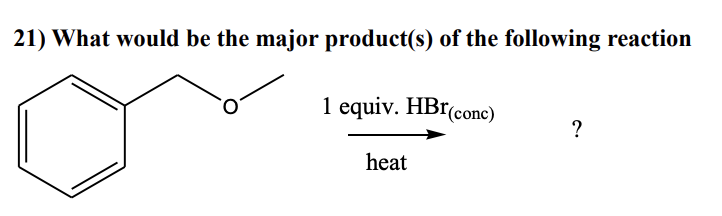
d) C6H5CH2Br + CH3OH
Which method would provide the best synthesis of ethyl isopropyl ether?
a) (CH3)2CHONa + CH3CH2Br →
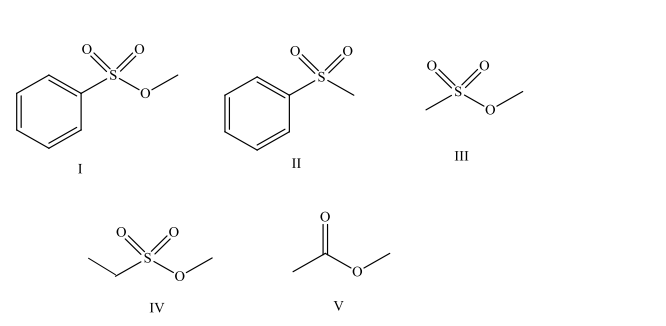
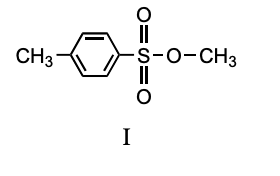
Which compound is a mesylate?
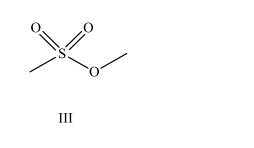
The major industrial process in use today to produce methanol is the:
) catalytic reduction of carbon monoxide
When nucleophilic addition to a carbonyl group occurs, the carbon attacked undergoes this hybridization change:
sp2—>sp3
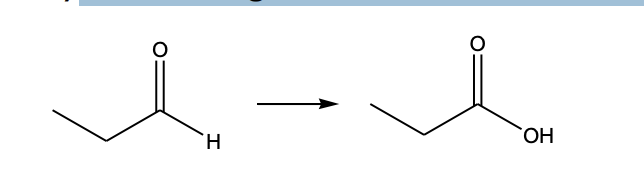
The following transformation would be considered a(n)?
b) oxidation
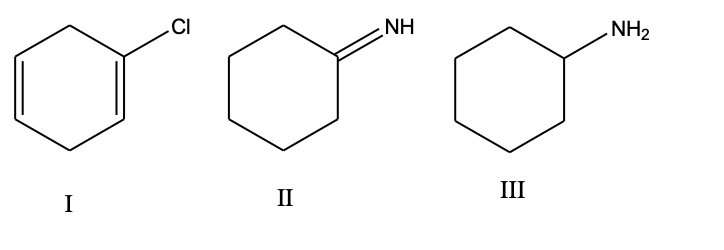
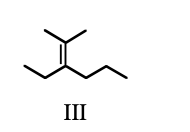
List the following compounds in order of increasing level of oxidation:
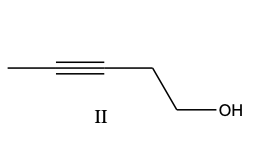
b) III, II, I
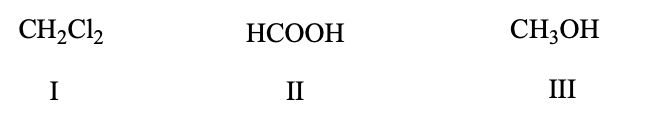
List the following compounds in order of increasing level of oxidation:
d) III, I, II

List the following alkyl halides in order of increasing ease of Grignard formation:
c) IV, II, I, III
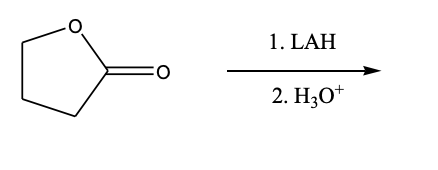
6) Which product is formed from the following transformation:
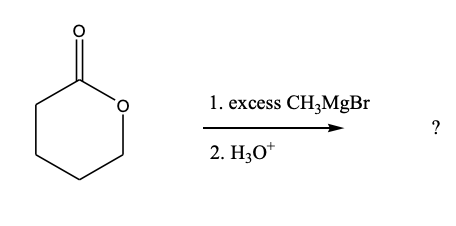
Which product is formed from the following transformation:
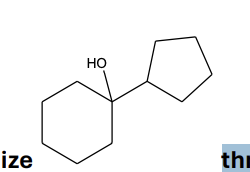
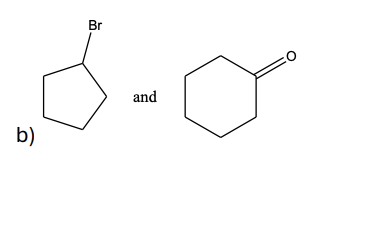
Your task is to synthesize through a Grignard synthesis. Which pairs of compounds listed below would you choose as starting materials?
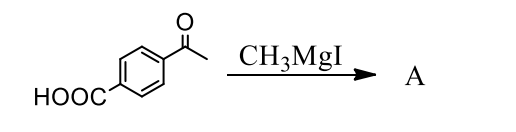
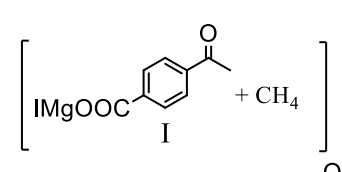
What is the principal product(s) formed when 1 mol of methylmagnesium iodide reacts with 1 mol of pacetylbenzoic acid?
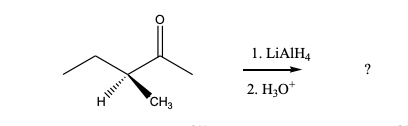
Predict the product(s) of the following reaction:
b) A mixture of I and III
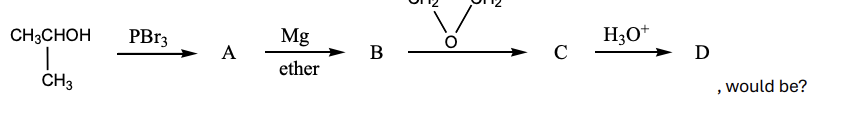
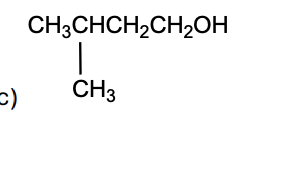
The final product, D, in the following reaction sequence
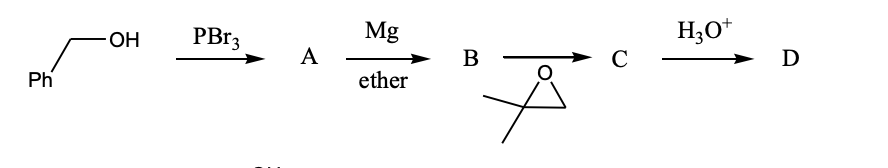
What would be the final product, D, in the following reaction sequence?
When oxidation from an alcohol to a carbonyl group occurs, the functionalized carbon undergoes this hybridization change:
d) sp3 → sp2
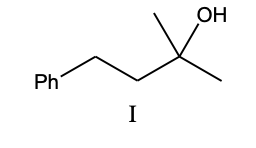
What is the product, A, that would be obtained from the following reaction sequence?
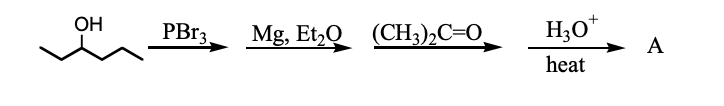
What is the product, A, that would be obtained from the following reaction sequence?
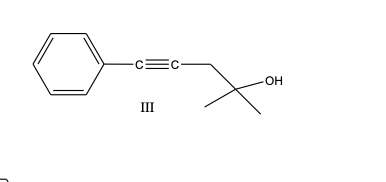
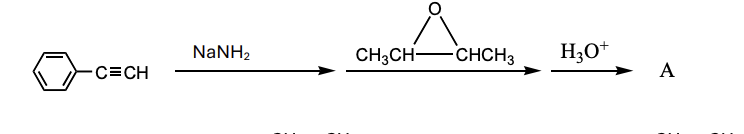
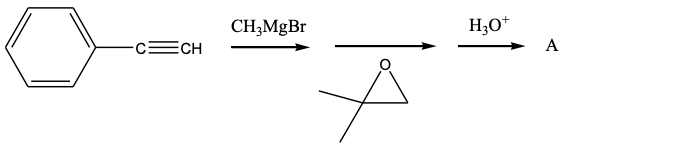
What is the product, A, that would be obtained from the following reaction sequence?
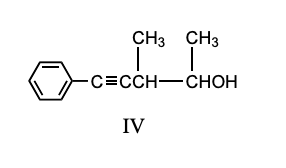
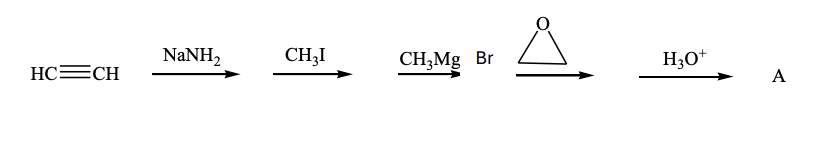
What is the product, A, that would be obtained from the following reaction sequence?
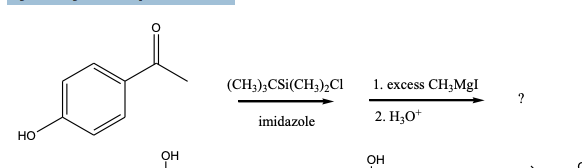
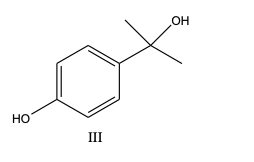
What is/are the principal product(s) formed when excess methylmagnesium iodide reacts with phydroxyacetophenone?
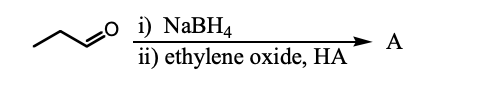
What would be the major product of the following reaction?
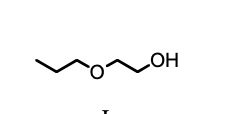
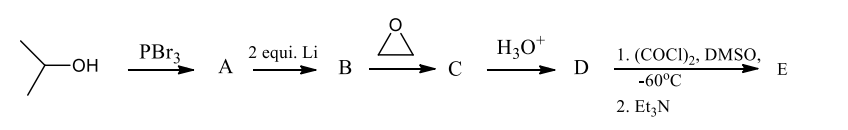
The final product, E, in the following reaction sequence is,
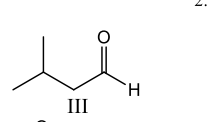
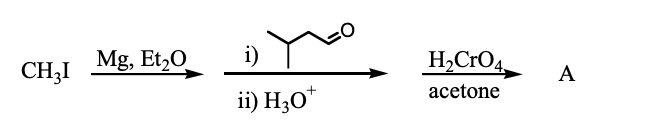
What is the final product of the following reaction sequence?
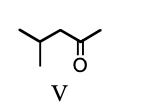
The reagent PCC in dry methylene chloride is successful in the selective oxidation of primary alcohols to aldehydes because:
c) hydration of the intermediate aldehyde is unable to take place in dry methylene chloride.
When 2-pentanol is treated with chromic acid
d) the color changes from orange to green
Which of these compounds cannot be used to prepare the corresponding Grignard reagent?
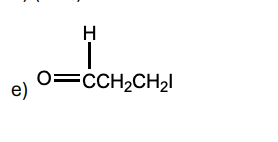
if the role of the solvent is to assist in the preparation and stabilization of the Grignard reagent by coordination with the magnesium, which of these solvents should be least effective?
CH3(CH2 )4CH3
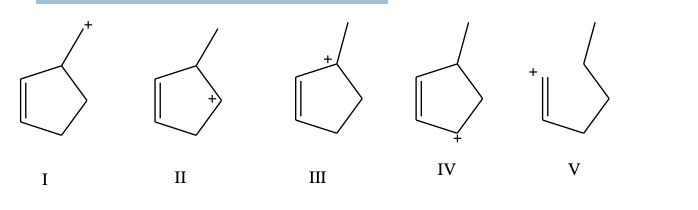
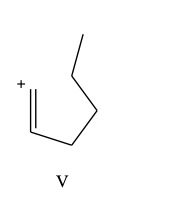
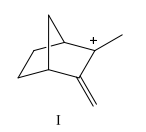
Which carbocation would be least stable?
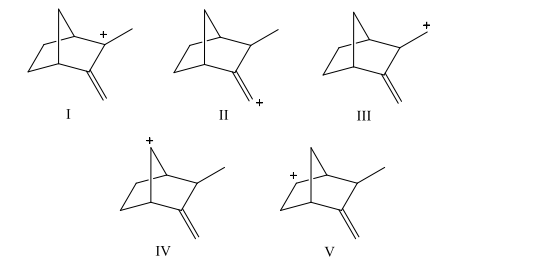
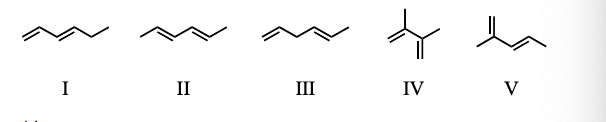
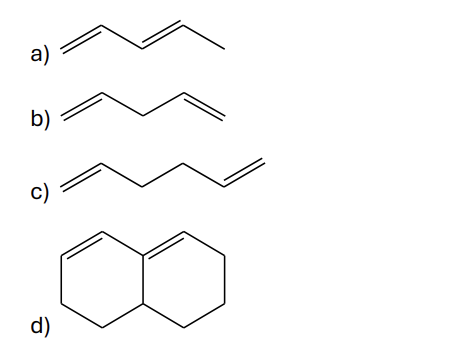
Which of the following dienes would you expect to be the most stable?
a) CH3CH=CHCH=CHCH3
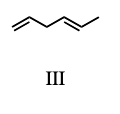
4) Which diene would be the least stable?
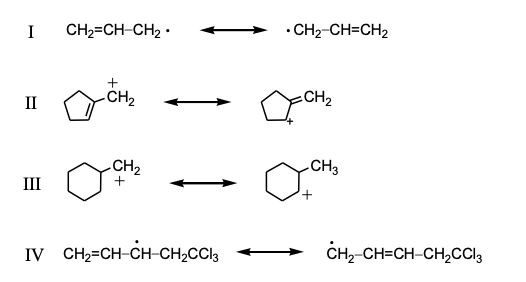
Which is not an example of resonance?
Select the structure(s) in which the multiple bonds are conjugated.
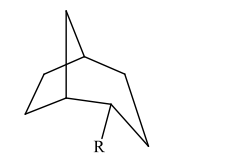
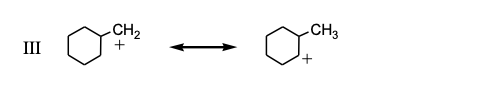
The substituent R on the bicyclic compound shown is considered to be?
c)endo
Which carbon-carbon bond in the following compound would you expect to be shortest?
a) I
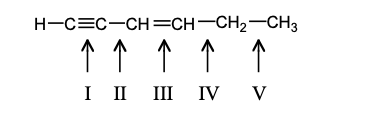
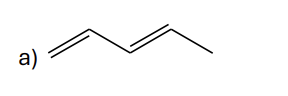
Which alkene would you expect to have the lowest heat of hydrogenation?
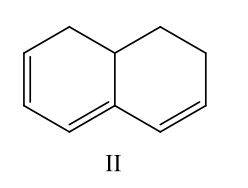
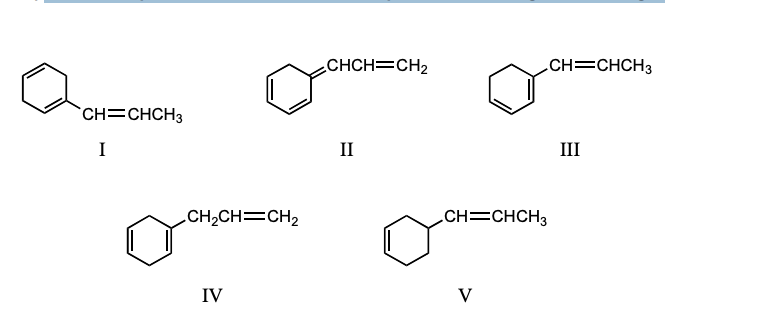
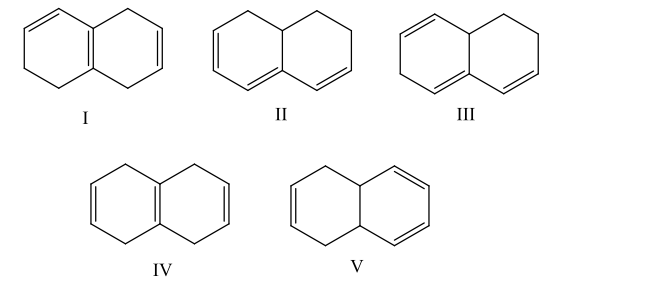
Which compound would have a UV absorption band at longest wavelength
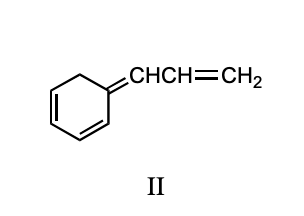
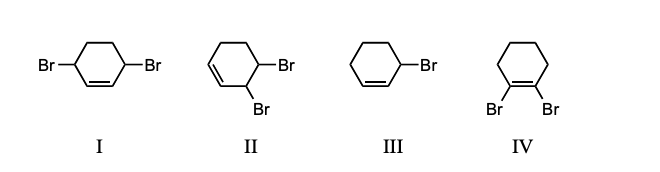
Ignoring stereochemistry, the 1:1 reaction of bromine with 1,3-cyclohexadiene at 25 C in the dark and in the absence of peroxide forms which of these?
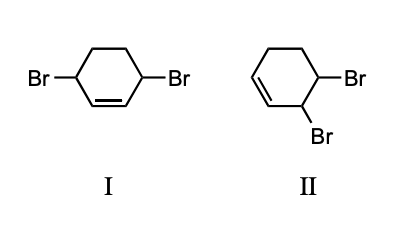
A thermodynamically controlled reaction will yield predominantly:
a) the more/most stable product
Which is an untrue statement concerning the Diels-Alder reaction?
d) Generally, the adduct formed most rapidly is the exo product.
Which of these dienes can undergo the Diels-Alder reaction?
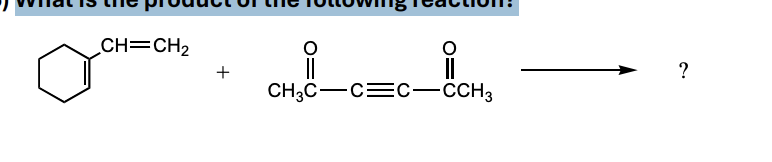
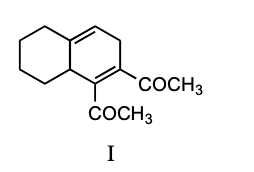
What is the product of the following reaction?
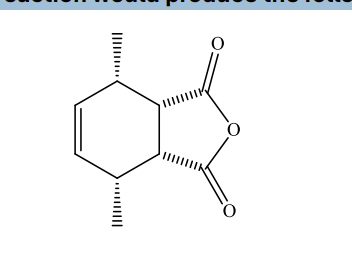
Which reaction would produce the following compound?
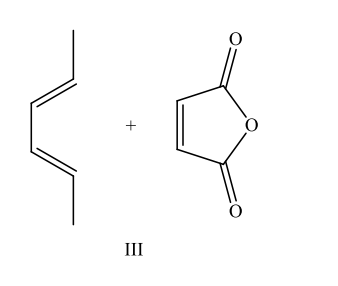
Which reaction would produce the following compound?
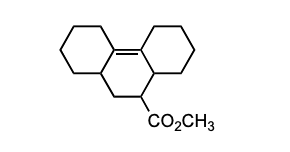
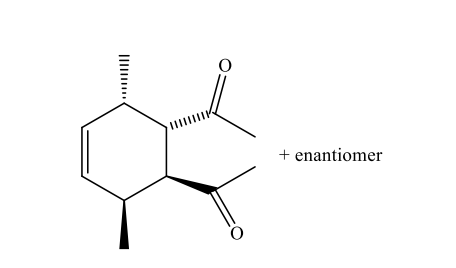
Which of the following pairs of compounds could be used as the basis for a Diels-Alder synthesis of the compound shown below?
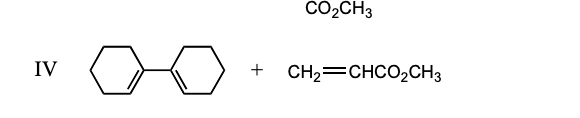
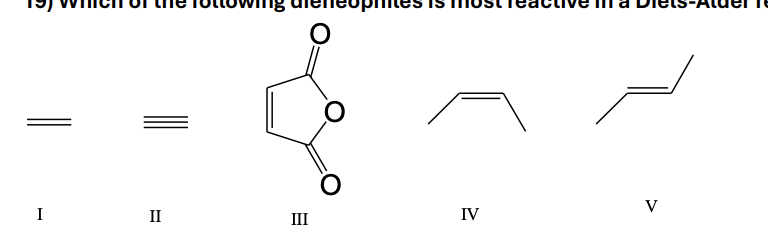
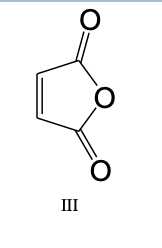
19) Which of the following dieneophiles is most reactive in a Diels-Alder reaction:
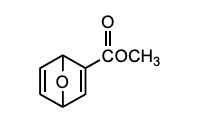
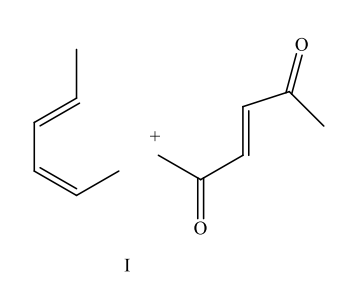
Which diene and dienophile would you choose to synthesize the following compound?
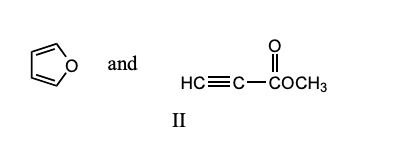
Which of these dienes is the most reactive in the Diels-Alder reaction?
c) 2,3,-Dimethyl-1,3-butadiene
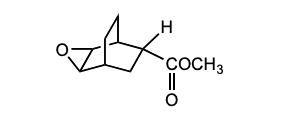
How would you synthesize:
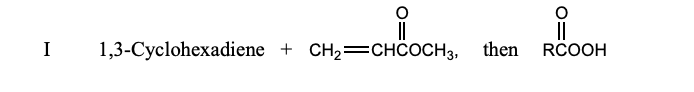
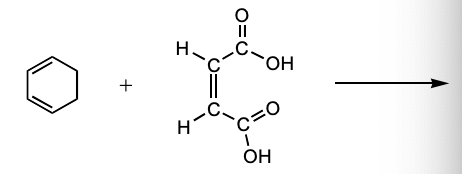
Which is the major product of the following reaction?
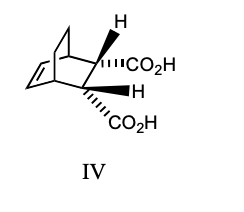
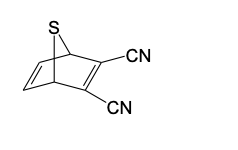
Which compounds could be used in a Diels-Alder synthesis of
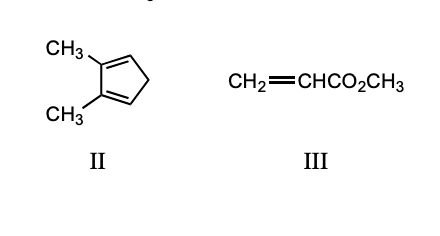
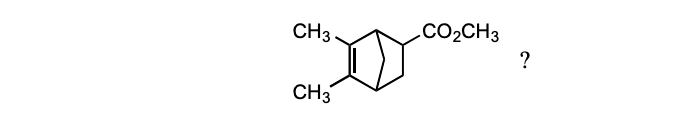
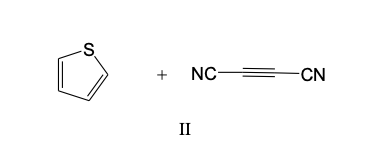
Which diene and dienophile would you choose to synthesize the following compound?
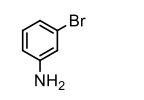
The correct name for the compound shown below is:
a) m-bromoaniline
Aniline is the name commonly assigned to:
b) Aminobenzene
Which dichlorobenzene might theoretically yield three mononitro products?
b) m-Dichlorobenzene
How many different dibromophenols are possible?
6
) Which is the only one of these reagents which will react with benzene under the specified conditions?
a) Cl2, FeCl3
Which reagent(s) would serve as the basis for a simple chemical test that would distinguish between benzene and 1-hexene?
b) Br2
In the molecular orbital model of benzene, the six p orbitals combine to form how many molecular orbitals?
a) 6
Which of the following is NOT true of benzene?
The carbon-carbon bonds of benzene are alternately short and long around the ring
We now know that the two Kekulé structures for benzene are related in the following way:
d) Neither of the two structures adequately describes benzene; benzene is a resonance hybrid of the two.
Which of the following statements regarding the cycloheptatrienyl radical is correct?
b) It is not aromatic.
) Which of the following statements regarding the cycloheptatrienyl anion is correct?
b) It is antiaromatic.

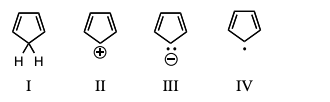
Which of the following would you expect to be aromatic?
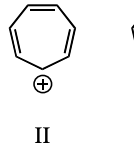
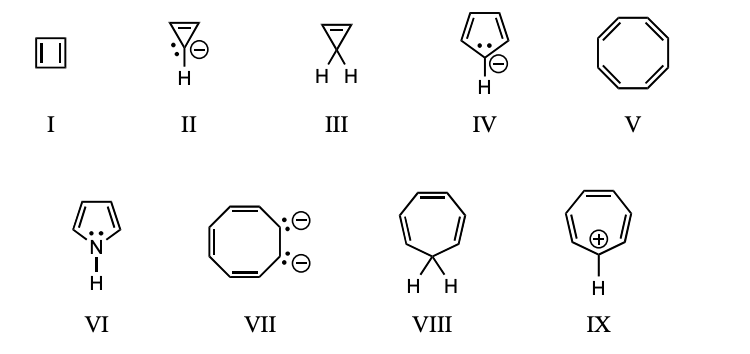
On the basis of molecular orbital theory and Hückel’s rule, which molecules and/or ions should be antiaromatic?
I,III,V
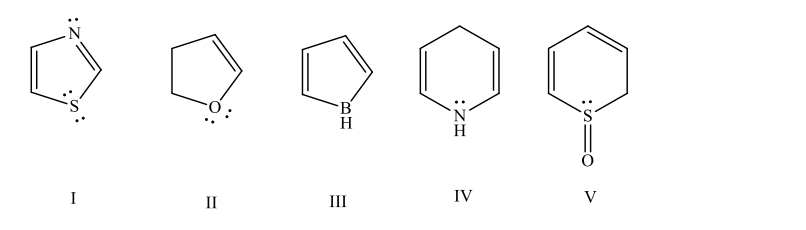
Which of these is an aromatic molecule?
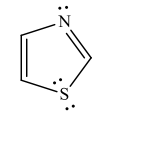
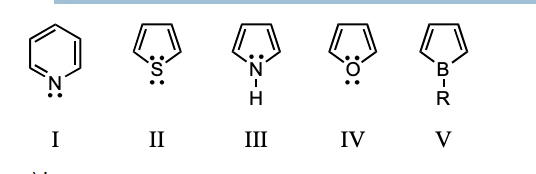
Which compound would you NOT expect to be aromatic?
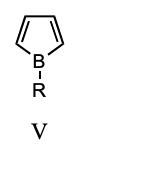
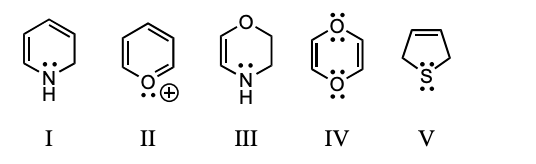
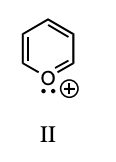
Which of these species is aromatic?
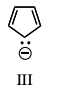
How many equivalent resonance structures can be written for the cyclopentadienyl anion?
5
19) How many resonances (signals) would be expected in the broadband proton-decoupled 13C spectrum of:
5
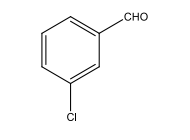
How many resonances (signals) would be expected in the broadband proton-decoupled 13C spectrum of:
7
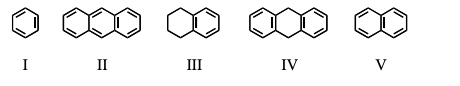
Which of these compounds absorbs at the longest wavelength in the UV-visible region?
B) II
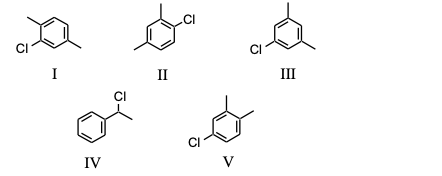
Which of the following substances, C8H9Cl, would exhibit five signals in its 13C NMR spectrum?
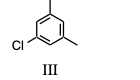
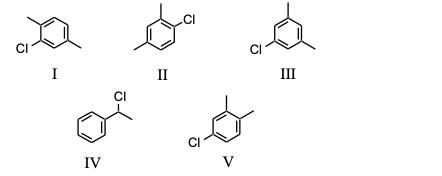
Which of the substances from Question #22, would exhibit six signals in its 13C NMR spectrum?
d) IV
![<p>Which reagent(s)/technique would serve to distinguish between azulene and bicyclo[5.3.0]decane</p>](https://knowt-user-attachments.s3.amazonaws.com/ec448468-9a4e-4099-8949-9b2a863807f0.png)
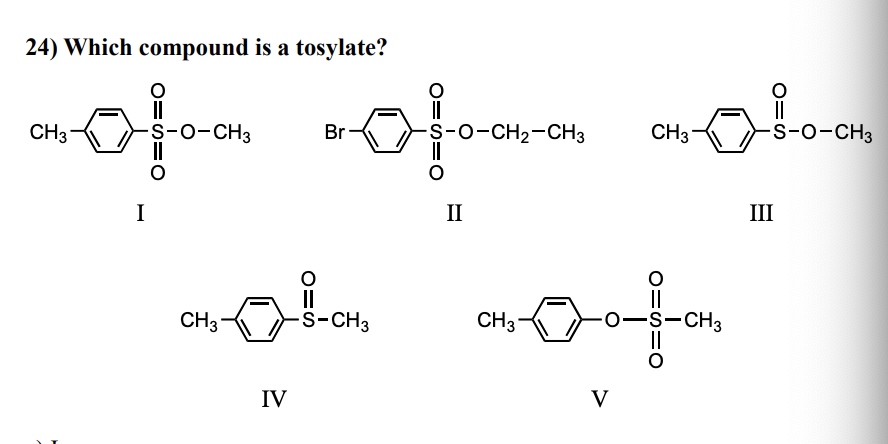
Which reagent(s)/technique would serve to distinguish between azulene and bicyclo[5.3.0]decane
c) NMR Spectroscopy
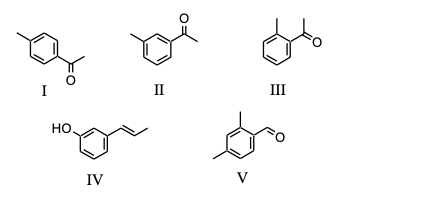
For which of the following substances, C9H10O, would the aromatic multiplet, in its 1H NMR spectrum, consist of only two doublets?
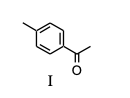
Which of the following would you expect to be aromatic?