Topic 1.5 - Kinetics
1/7
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
8 Terms
What must particles do in
order to react?
Collide with sufficient energy (activation energy)
and the correct orientation
Do most collisions result in
a reaction?
no
Define Activation Energy.
The minimum energy that particles must collide
with for a reaction to occur
Draw a labelled Maxwell-Boltzman
Curve. Label average energy, activation
energy and most probable energy.
Draw in a different colour the effect of
increasing temperature
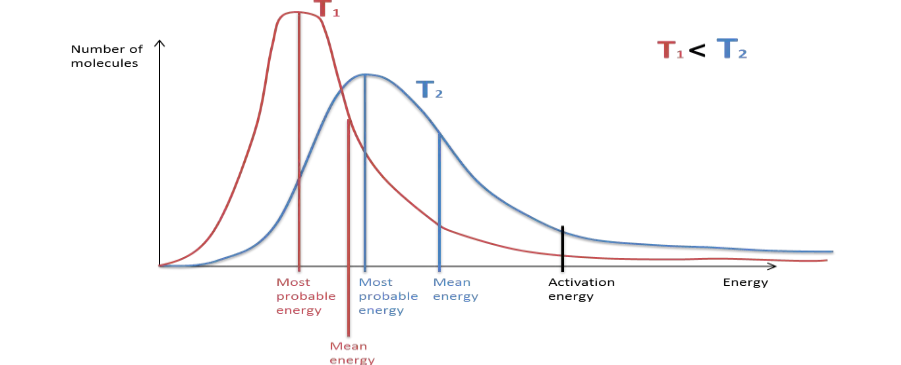
What is the effect of
increasing temperature on
rate of reaction? why?
Increasing temperature → increased rate of
reaction
Much higher proportion of particles have energy
greater than the activation energy → many more
successful collisions per second →increased rate
What is the effect of
increasing
concentration/pressure on
rate of reaction? why?
Increased concentration/pressure → increased rate of
reaction
There are more particles in a given volume → more frequent
successful collisions → increased rate
What is a catalyst?
A substance which increases the rate of reaction
but is not used up in the reaction
How do catalysts work and
how do they increase the
rate of reaction?
Provide an alternative reaction pathway (one with a lower
activation energy)
Lowers activation energy, so more particles have energy >
activation energy, so more frequent successful collisions, so
increased reaction rate