Chemistry unit 2 the atom
1/40
Earn XP
Description and Tags
Pain and suffering. Oh and also Average atomic mass Nuclear decay (be able to write decay equations) Half-life problems Dalton's Atomic Theory Models of the atom (plum pudding, nuclear, Bohr, quantum) Properties of light (wavelength, frequency, energy, speed Quantum Model
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
41 Terms
Electrostatic force
Force of attraction between positive protons and negative electrons
force of repulsion between positive protons
Acts over long distances (between electron cloud and nucleus)
Strong force
Force of attraction between protons and neutrons
Acts over short distances (between neighboring protons and neutrons)
What is a quanta?
the minimum amount of energy that can either be lost or gained by an atom.
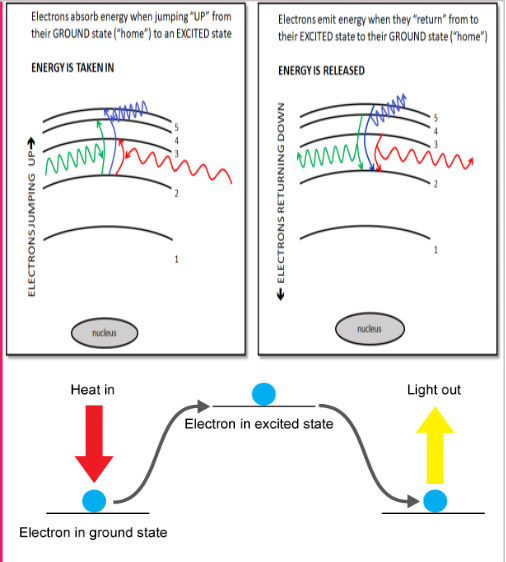
When an electron jumps from its ground state up into an excited state does the atom absorb or release energy?
absorbs
Alpha decay
removes two protons and neutrons (4/2 He)
moves to the left on the periodic table
Ex: (238/92 U)→(234/90 Th)+(4/2 He)
Beta decay
Turns a neutron into a proton
When the neutron splits apart it gives off a proton and a beta particle. The beta particle has 0 mass but a -1 number in the bottom
(14/6 C)→(14/7 N) +(0/-1 e)
What is the goal of Alpha and Beta decay?
Both have too many neutrons, the goal is to get an equal amount of protons and neutrons. Alpha is used when there is a lot more neutrons than protons, beta is used in smaller numbers.
Gamma decay
Nucleus in excited state→high energy gamma ray emitted
only gets rid of energy
(12/6 C) → (12/6 C) +y
nucleus changes from a higher energy state to a lower energy state through the emission of electromagnetic radiation (photons)
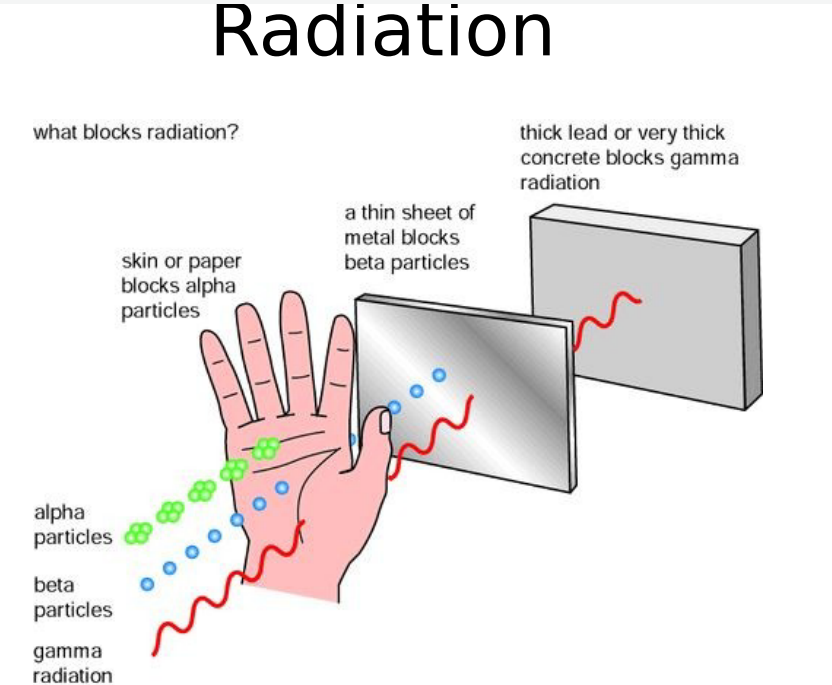
What blocks radiation
Nucleur fission
heavy nucleus divides to form smaller, more stable nuclei, and releases large amounts of energy
many isotopes of heavy elements undergo fission if bombarded by high energy neutrons
additional neutrons are also produced that can induce fission in other nuclei, which in turn produces more neutrons, and so on. A nuclear chain is created.
Fission is used in nuclear weapons and nuclear reactors
A form of alpha decay
Nuclear fusion
lighter nuclei combine to form larger, more stable nuclei
fusion reactions release much more energy than fission reactions
fusion creates the energy in the sun
Band of stability
Whenever the difference between neutrons to protons within a nucleus is significant enough an isotope is radioactive
the number of the protons is very high above 82, so the ratio of neutron and proton becomes less or greater than the one; therefore, the ratio becomes unstable.
Band of stability ends at 82, numbers above 82 are unstable
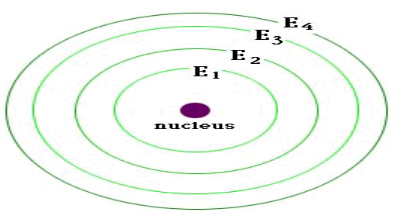
Energy levels
Lowest energy level is closest to the nucleus. (E1)
The higher the energy level the further it is from the nucleus.
Quantum theory
a mathematical equation that describes the most probable positions of electrons in an atom
Solutions to Schrodinger’s equation, gives the probability of finding an electron
electrons do not travel in neat orbits around the nucleus as Bohr said; electrons exist in regions called electron clouds or orbitals
Orbitals
orbitals are 3 dimensional regions around the nucleus where an electron is most likely to be found
each orbital has a unique amount of energy
electrons farther away from the nucleus have greater amounts of energy
quantum numbers
set of “coordinates” that describes the position and properties of electrons
Quantum numbers are the solution to Schrodinger’s equation
quantum numbers tell us the:
Shape of the orbitals (regions around the nucleus where electrons are likely to exist)
Distance from the nucleus (and therefore the amount of energy electrons have)
Thing of quantum numbers as the address for each electron
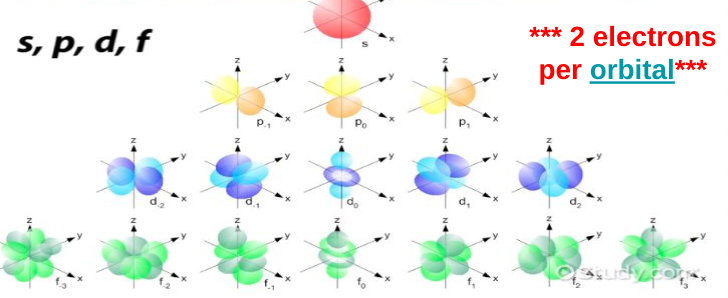
S sublevel
Max 2 electrons
Sphere shape
1 configuration
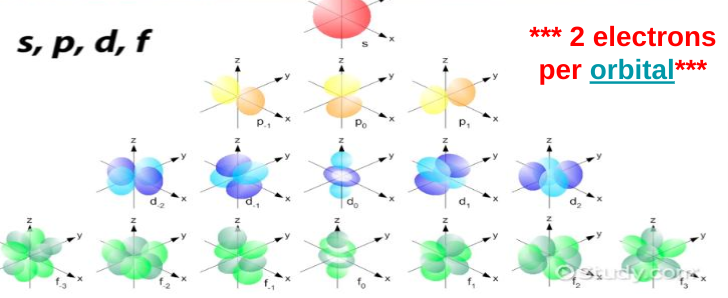
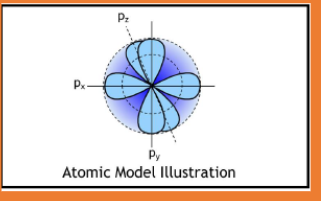
P sublevel
Max 6 electrons
3 3D orientations
Dumbbells
6 electrons because there are 2 electrons in each configuration (2 in the first way you can place a dumbbell, 2 in the second unique way you can place a dumbbell, and 2 in the third unique way you can place a dumbbell)
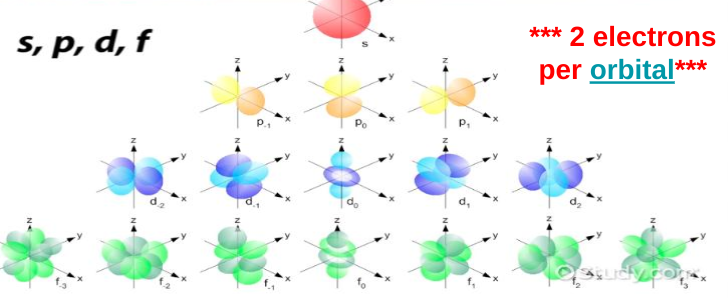
D sublevel
set of clovers
5 3D orientations
10 electrons max
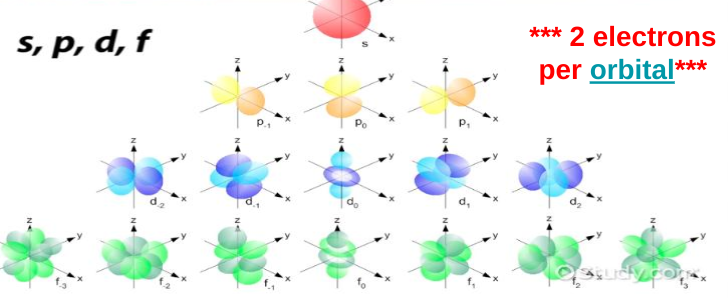
F sublevel
flower petals
7 3D orientations
max 14 electrons
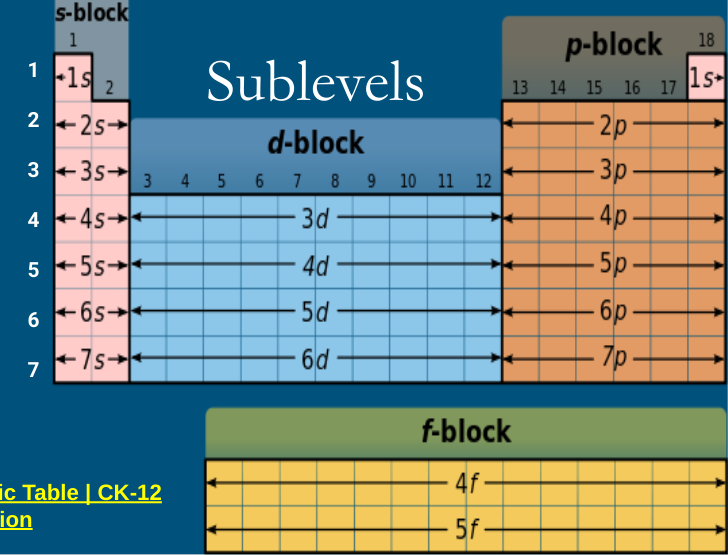
Sub levels and special rules
Helium counts as part of the s-level while still being a noble gas
you do n-1 for the d block
you do n-2 for the f block
When looking at the total number of electrons in level 3 don’t forget to count s, p, AND d. Even if the d block comes after 4s, it still counts as part of level 3 because it starts with 3
Ex: (3s² 3p^6 4s² 3d^10) total number in level 3: 18
Valence electrons
you can tell the number of valence electrons by looking at the column number (once you hit the P block just remove the one and use the number in the ones place as the number of valence electron)
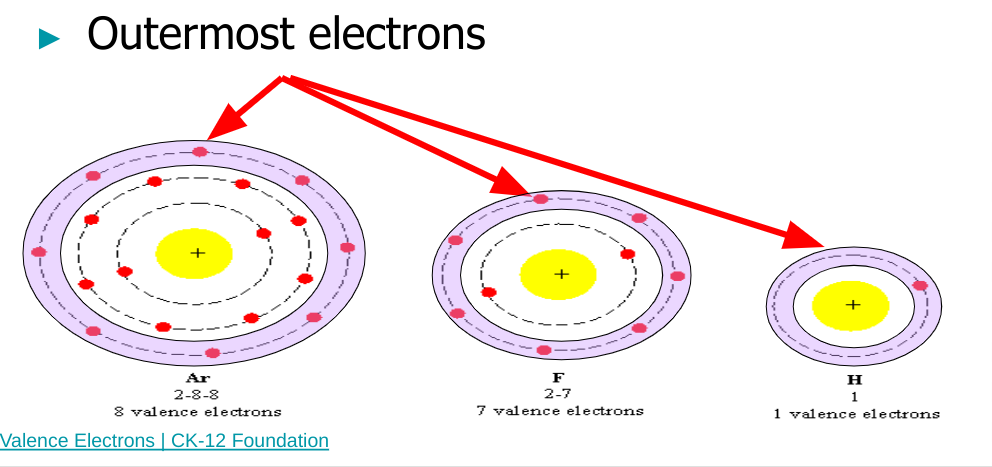
ions using electron configuration
An element may resemble a noble gas when you look at its electron configuration because it has either gained or lost electrons to get a perfect 8 in its outer orbital
If the outer orbital has 4 electrons, the element could either gain or lose 4
Fl: 1s²2s²2p^5 → 7 electrons in level two so it gains another to reach 8→ Fl^-1: 1s²2s²2p^6
Aufbau principle
The Aufbau principle, also called the Aufbau rule, states that in the ground state of an atom or ion, electrons fill subshells of the lowest available energy, then they fill subshells of higher energy. For example, the 1s subshell is filled before the 2s subshell is occupied.
Pauli exclusion principle
if two electrons occupy the same orbital, they must have opposite spin
Hunds rule
Electrons will pair up in an orbital only when all orbitals in the same sub level have one one electron
When single electrons occupy different orbitals of the same sub level they all have the same spin
orbital diagrams
Arrows represent the electrons spinning
the amount of lines must match the number of orientations
There must be up arrows in every line before down arrows can be paired with the up arrows
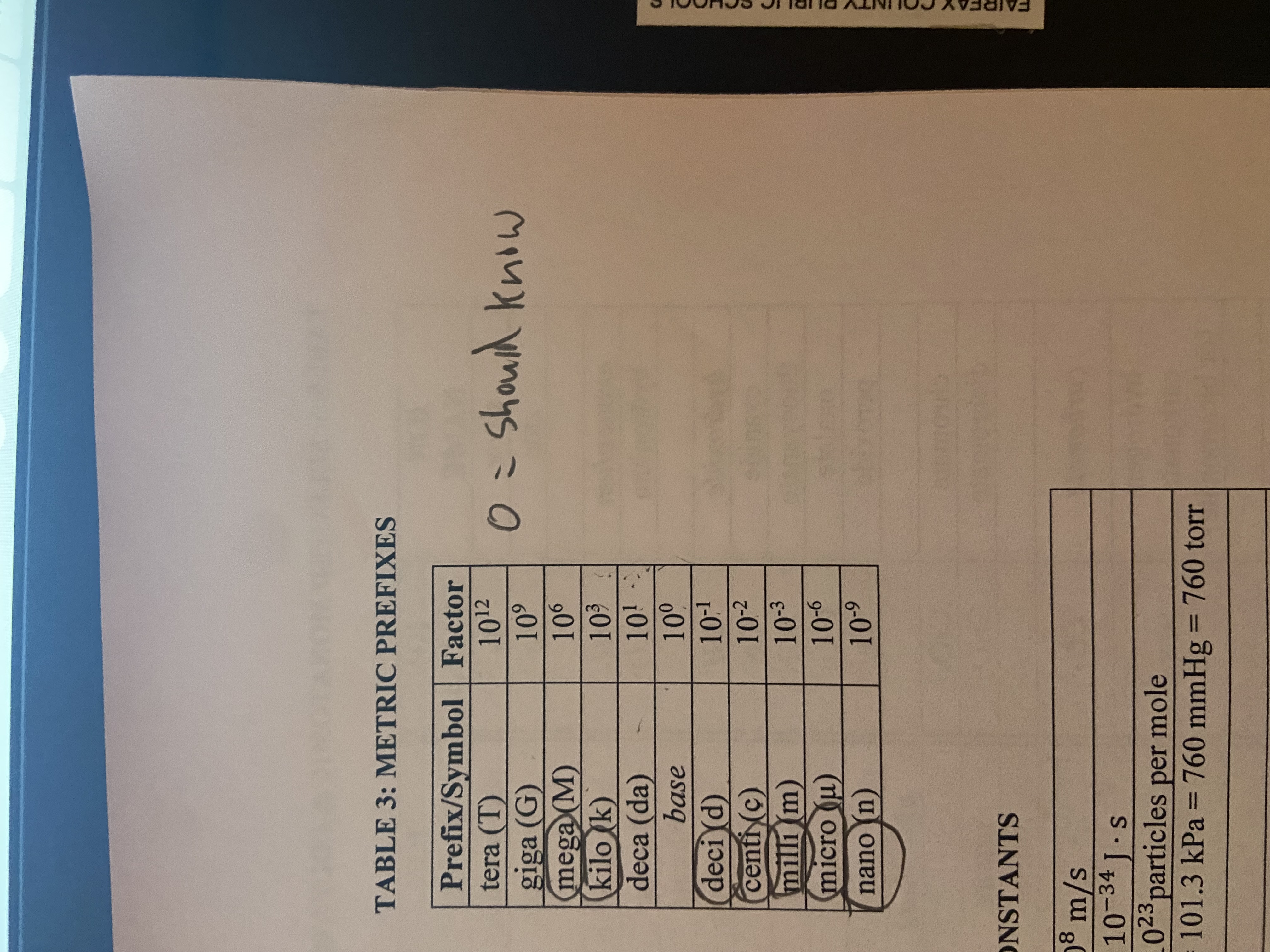
Units you need to know
learn deca too even if its not circled
Democritus
400 BC
Proposed the idea of atomos, meaning indivisible
philosopher not a scientist
no experimental evidence only thought experiments
Dalton
1803
Billiard ball model, atoms are solid indestructible units
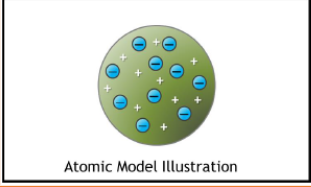
Thomson
1897
Plum pudding model
solid atoms consist of a uniform, positively charged substance containing small negatively charged electrons in it
Atoms consist of negative electrons embedded in positively charged mass
Charges are balanced, atom is neutral (no net charge)
Cathode ray experiment→discovered that there are electrons.
Rutherford
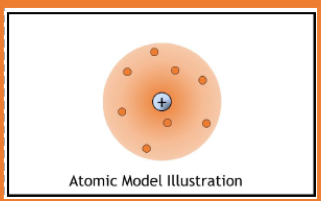
1911
nuclear model
A small, dense, centrally located positive region (nucleus) surrounded by negatively charged electrons in empty space
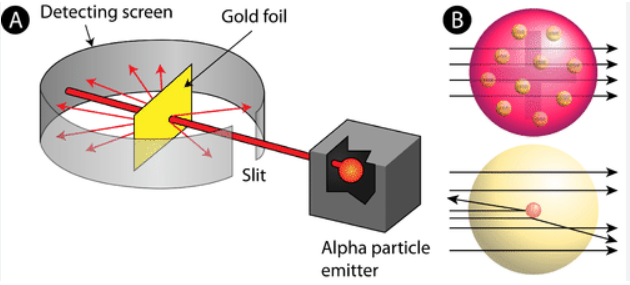
Gold foil experiment, when some atoms bounced back it proved that the atoms were mostly empty space, however they had something solid in the middle
Bohr
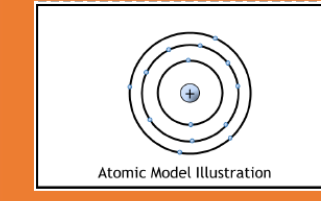
1913
Planetary model
Electrons orbit the nucleus at certain specific distances and have specific energies (ie the closer the electron orbits the nucleus, the lower the energy, the farther away, the higher)
Shrodinger
1926
probability model distribution
This model includes protons and neutrons (held together by strong force) in the nucleus surrounded by electrons in clouds (orbitals- representing the probability of finding electrons here)
All equations you need to know (regarding energy and light)
c=λv, c=speed of light (constant), λ=wavelength in m, v=frequency in /s
E=hv, v=frequency, h=Planck's constant, E= energy of one light photon
1 Angstrom (Å)=1.0 × 10-10m
1Hz=1/s
How does an atom give off color
You add energy (photons)
Electron gets excited
Electron moves up in energy levels
Electron moves back to original position→electron emits energy in the form of light
What colors have the highest energy, frequence, biggest wavelength etc?
Violets have the highest energy and frequency, and the smallest wavelength
Reds have the lowest energy and frequency and the biggest wavelength
All waves travel at the same speed (the speed of light)
Why can’t a single atom of hydrogen produce all four hydrogen spectral lines simultaneously?
Because hydrogen only has 1 electron, and there can only be 1 spectral line per electron.
Considering the previous statement, how is it we can see all four colors form a hydrogen gas discharge tube simultaneously?
There are multiple hydrogen atoms, each resulting in a certain wavelength of light. There are only 4 types of waves that hydrogen can emit, each of them with a unique amount of energy being emitted. You can see all 4 using a prism but you can only see all 4 because there are multiple atoms and therefore multiple electrons, just one electron would result in only one color.
How to calculate average atomic mass?
(percent abundance in decimal form)(amu)+(percent abundance of second isotope in decimal form)(amu) and so on
Half life equations
m^i/2^n=mf
T=(t1/2)(n) n=number of half lives