CHEM 105a LAB FINAL
1/261
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
262 Terms
Exp. 1 (density of liquids)- Objective
To determine the mass % concentration of an unknown NaCl solution by comparing the density of the unknown solution to the densities of multiple solutions of known mass % concentration
how: measure mass and volume of ""known:solutions to find density
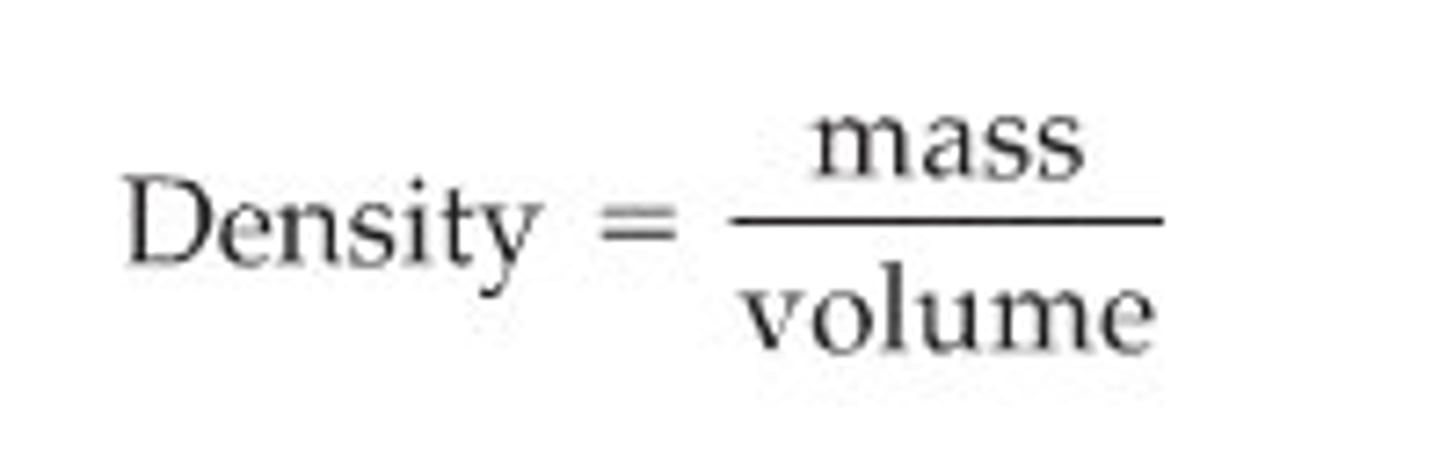
What is mass percent?
The percent by mass of a component of a mixture or of a given element in a compound
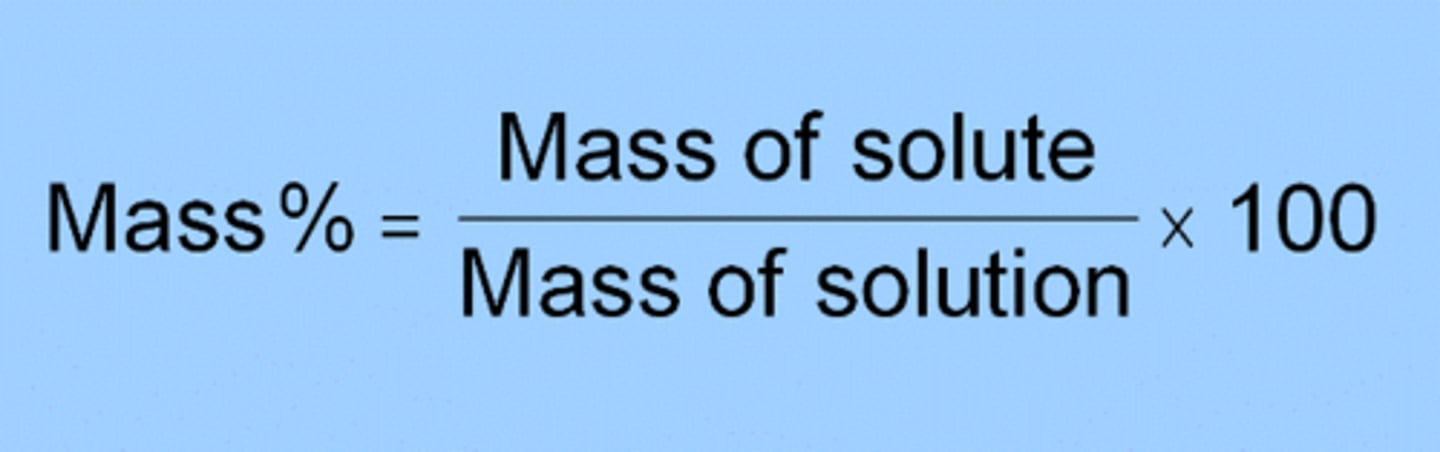
Exp. 1 (density of liquids)- Learning objectives
- accuracy, precision, random error, systematic error
- measure and dispense liquids with graduated pipets and burets
- measure with analytical and top loading balances
- results should be repeatable
- relationship between solute concentration and solution density
2 types of instruments to measure mass
Top-loading balance: Easier to use and can weigh larger samples
Analytical balance: More precise

2 types of instruments to measure volume
Graduated pipet and Buret
*Both are used when the volume of liquid being transferred must be measured accurately and precisely
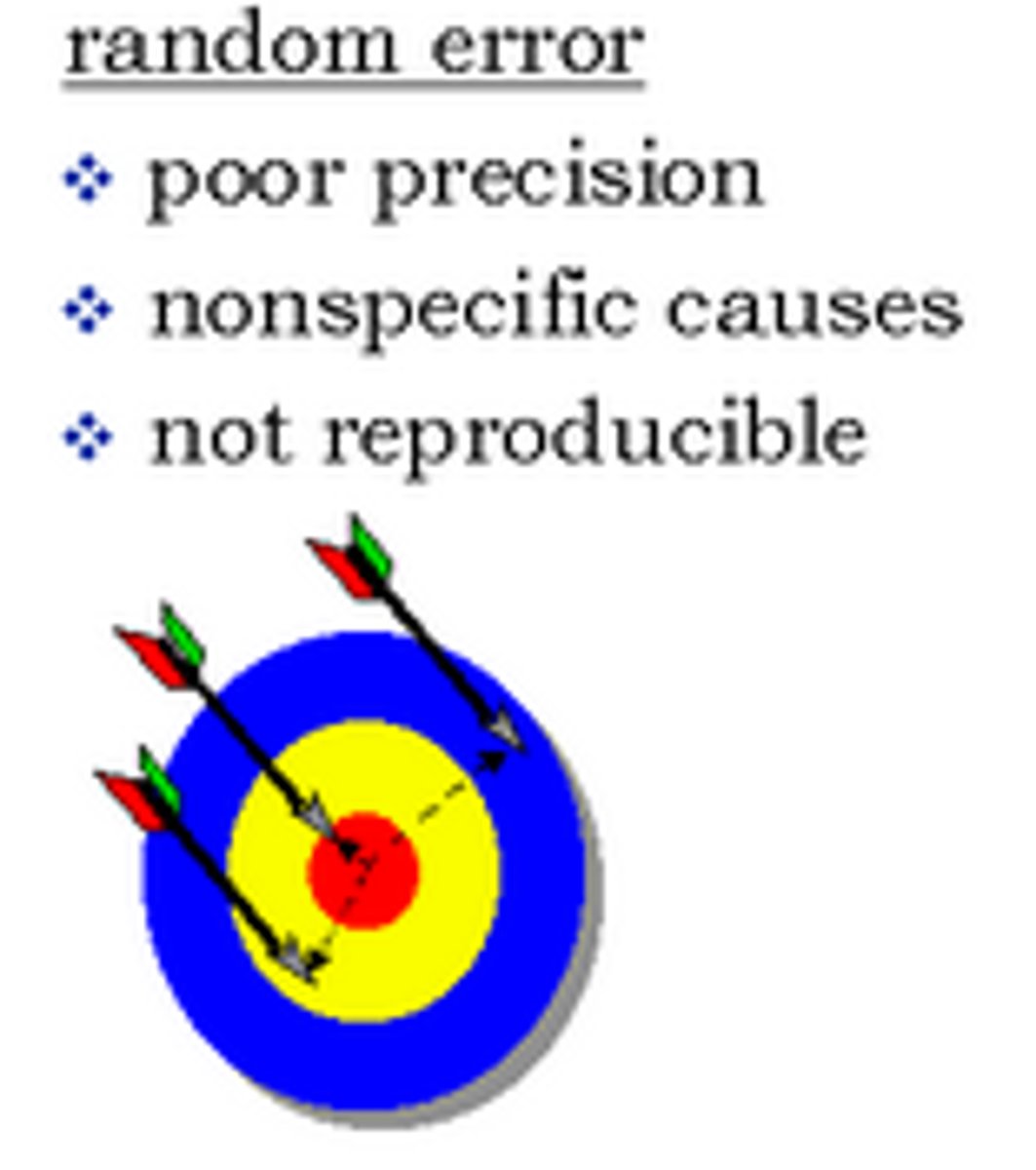
Random error
Measurement that lacks precision, but may cluster around true value.
ex. Equal opportunity of last digit being too high or too low
Systematic error
Error that is skewed in one direction
ex. poor experimental design, poorly calibrated instruments, or user error

Accuracy
How close a measurement is to the true value
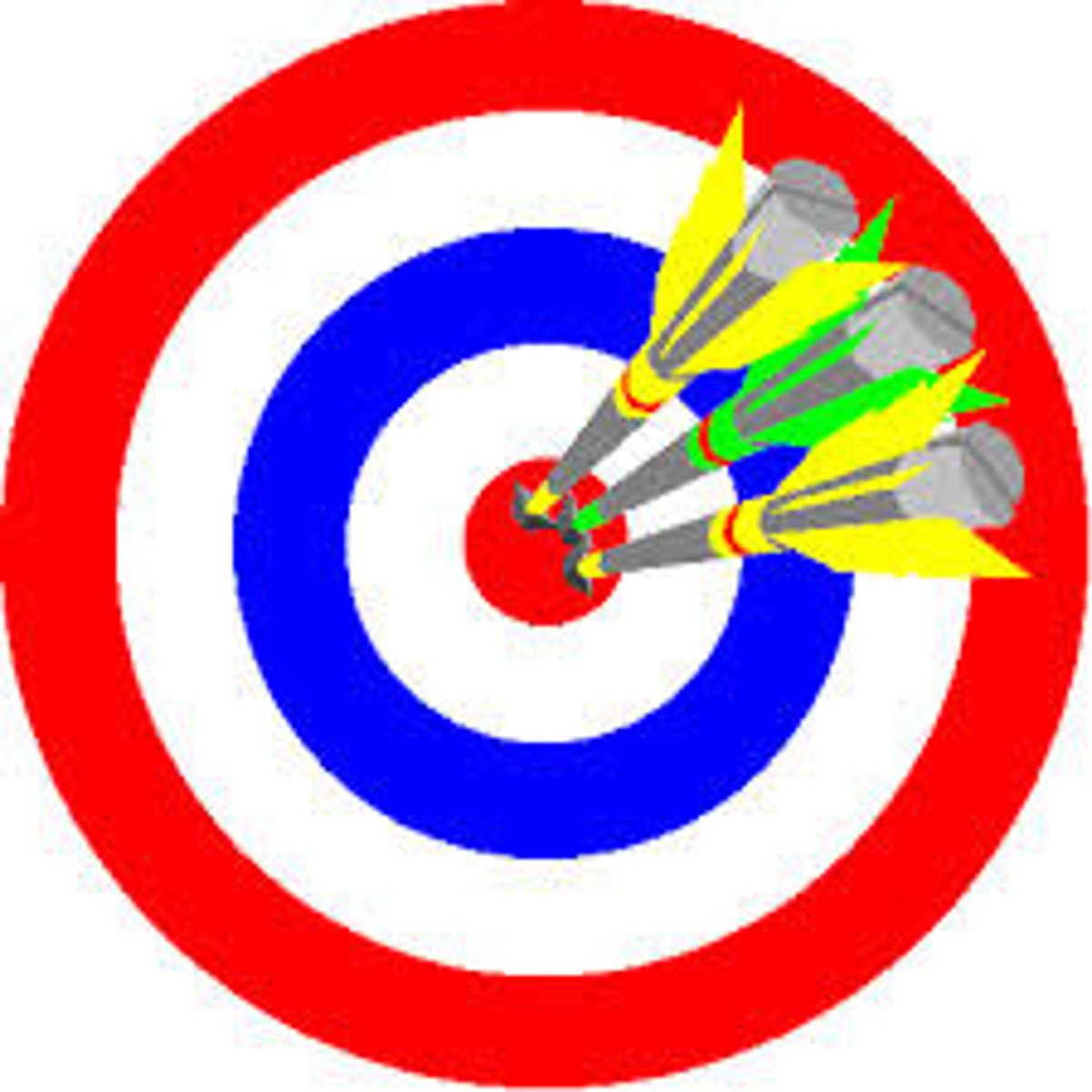
How can you measure accuracy?
Calculating percent error
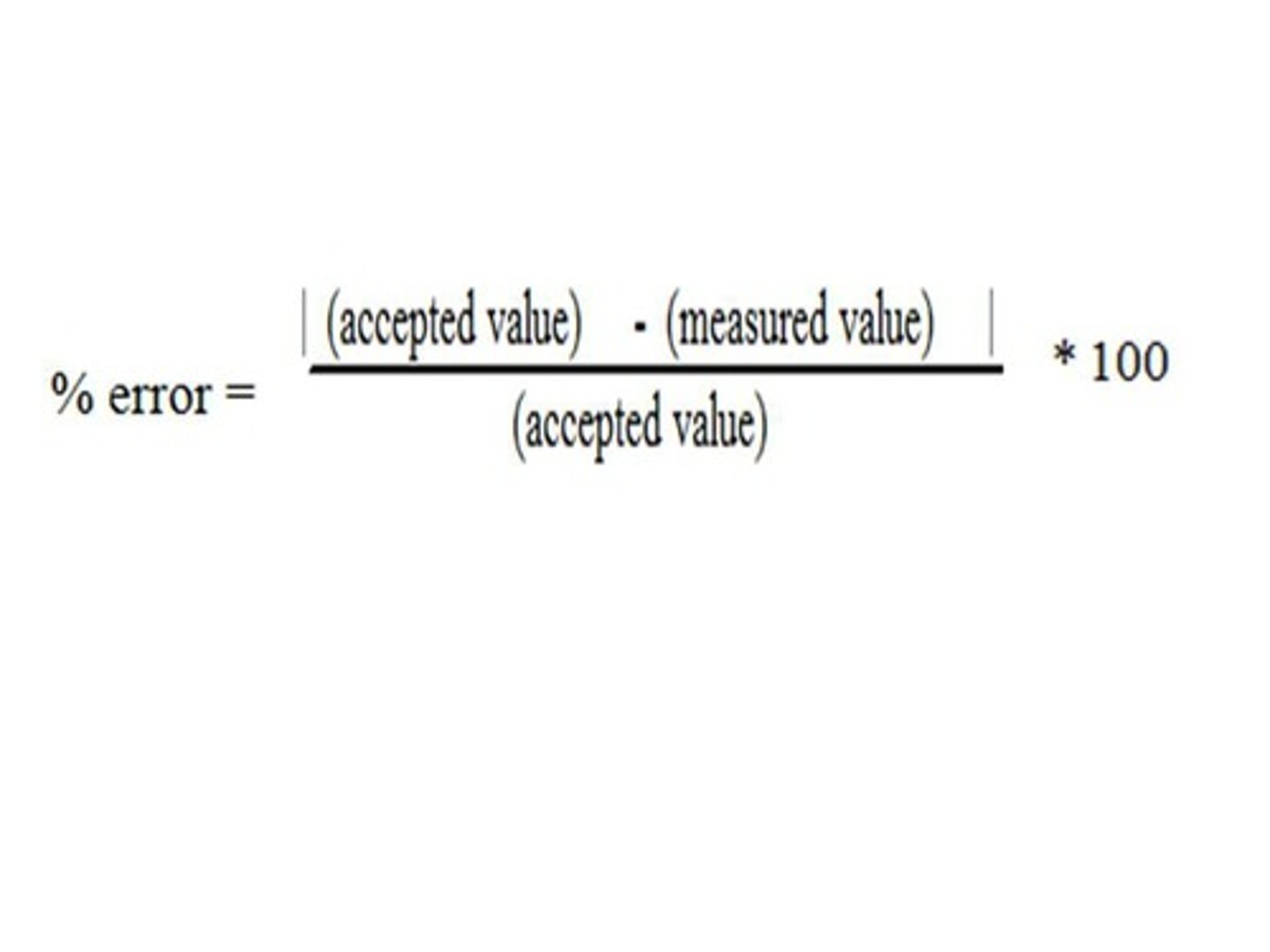
Percent error
The percent that a measured value differs from the accepted value
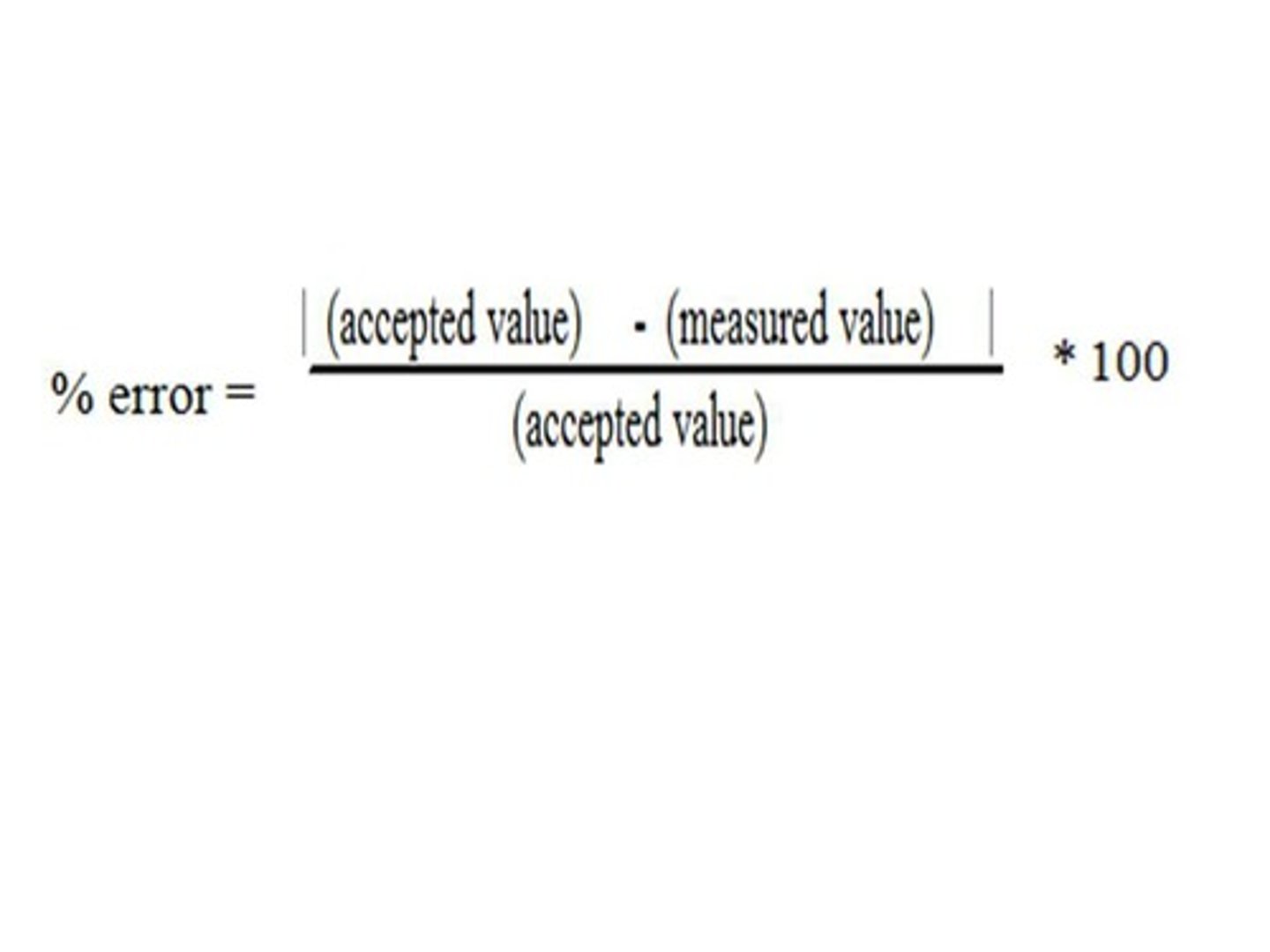
Precision
2 definitions:
1. The number of sig. figures an instrument can measure
2. The measure of repeatability of results
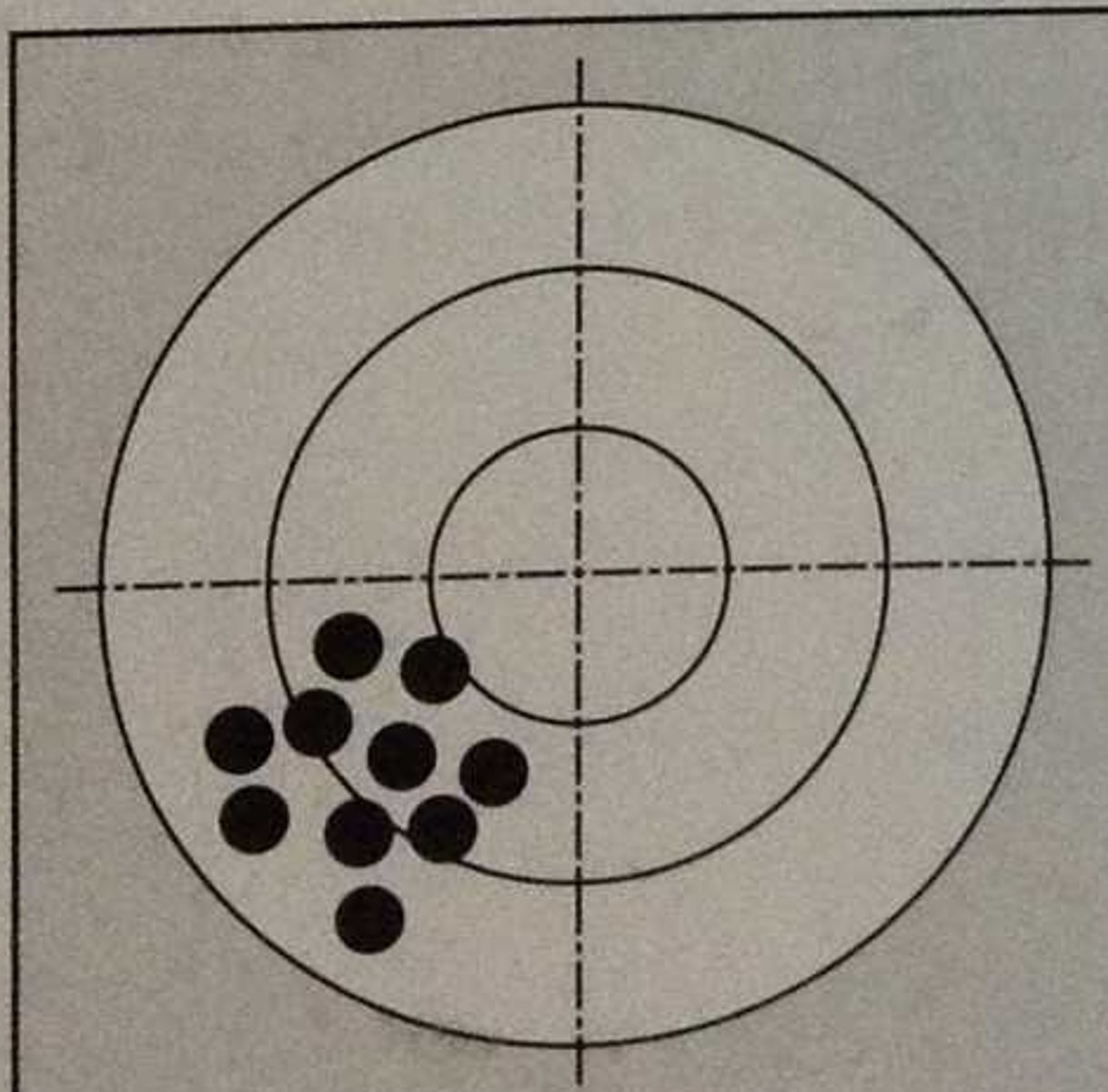
How can you measure precision?
Standard deviation or (percent) standard deviation
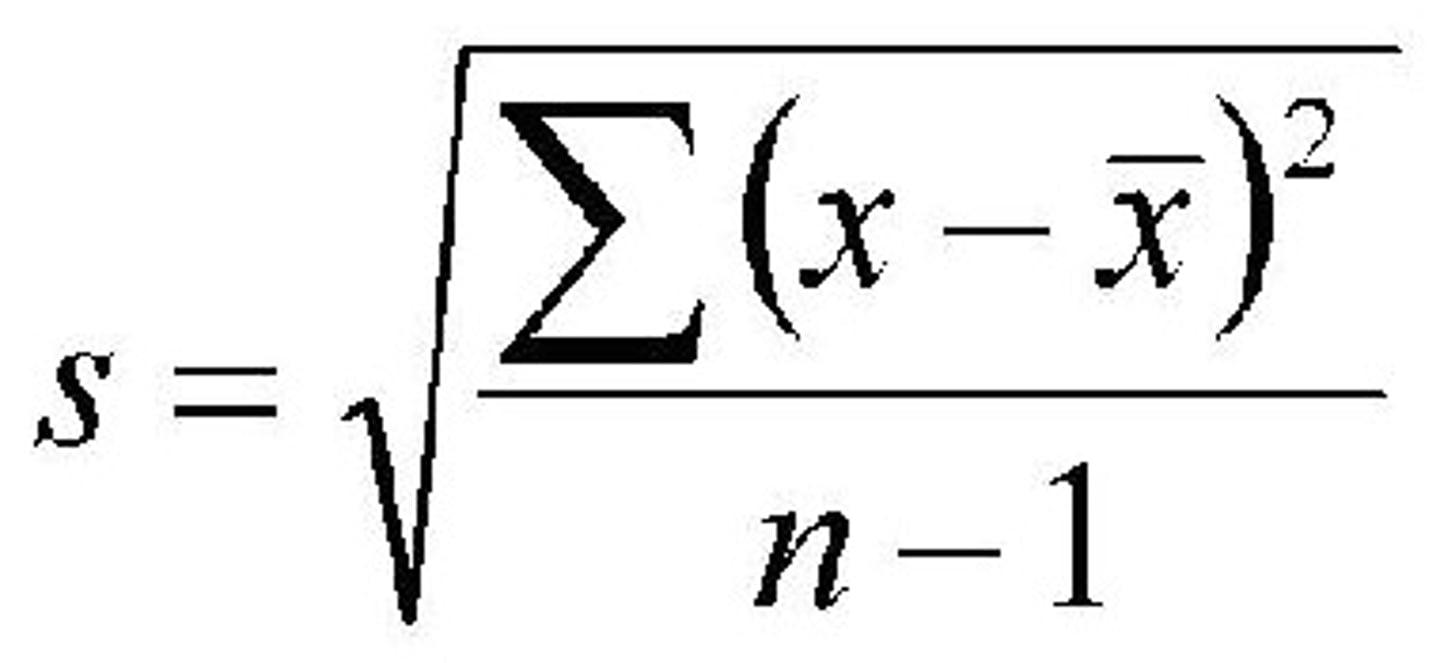
Standard Deviation
A computed measure of how much scores vary around the mean score
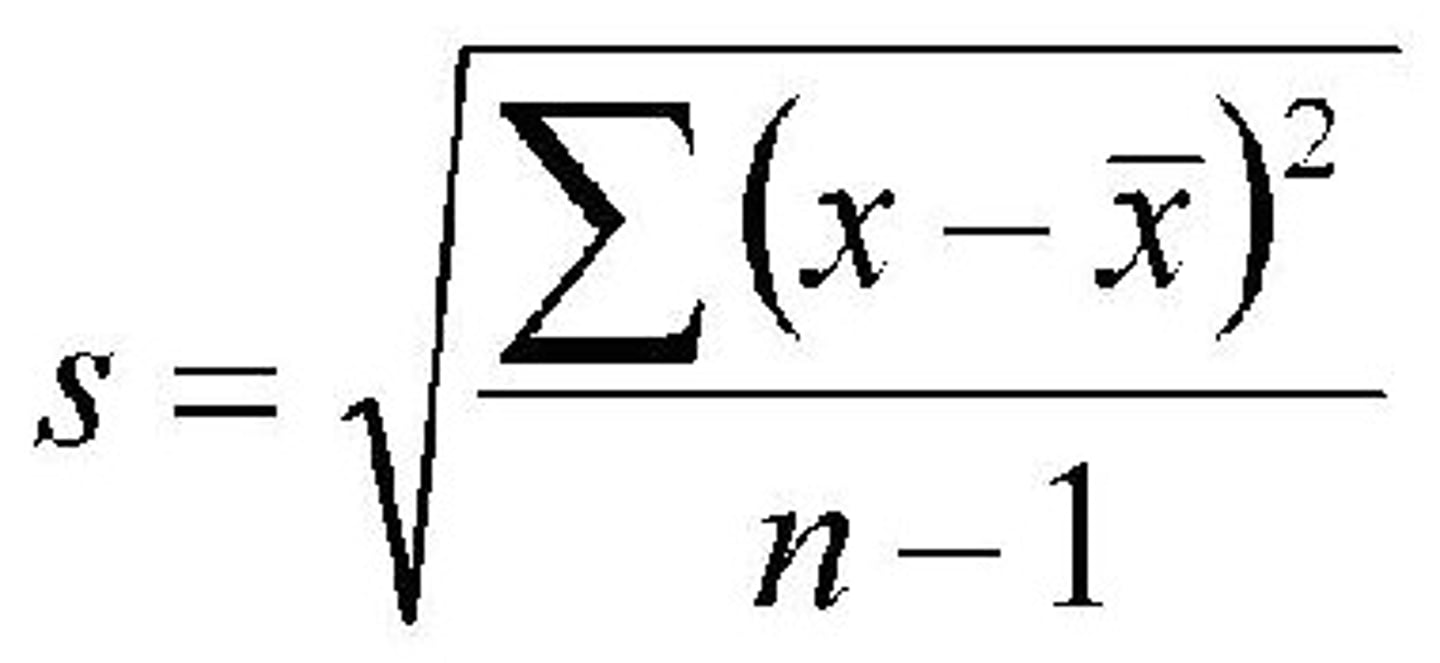
Exp. 1 (density of liquids)- Procedure
Determine density of 6 NaCl solutions in 2 separate trials --> measure mass and volume
Graduated pipet trial
1. Measure 5mL of solution
2. Find mass using "weigh by difference"
3. Measure temperature (C)
Buret trial
1. Measure 5mL of solution
2.Find mass using "weigh by difference"
3. Measure temperature (C)
Find densities of 5 DI water samples
Exp. 1 (density of liquids)- Out of the total solutions, how many were known and unknown?
5 known NaCl solutions
1 unknown NaCl solutions
Exp. 1 (density of liquids)- What method was used to measure mass of solutions?
"Weigh by difference"
Exp. 1 (density of liquids)- What do you do if you have excess solution? Took too much?
DO NOT pour into stock bottle again
(just share with another student or dispose into a waste container)
Exp. 1 (density of liquids)- Final calculations
1. Calculate density of each solution PER trial
2. Calculate average and standard deviation of density of DI water
3. Find percent error of average density of DI water and known density
4. Graph density vs. concentration of known NaCl solutions
5. Determine mass % concentration of unknown NaCl solution and include error of calculation
Exp. 1 (density of liquids)- Discussion and error
1. Understand which method has best results for density of water (consider accuracy and precision)
2. Offer explanations for the error in density of water
Exp. 2 (gravimetric analysis)- Objective
To determine the amount of phosphorus in a fertilizer designed for home gardening by the method of gravimetric analysis

Exp. 2 (gravimetric analysis)- Learning objectives
- Find phosphorus content of a solid using gravimetric analysis
- Separate an insoluble solid from a solution using vacuum filtration
- Mass percent composition
Gravimetric analysis
The formation and isolation of a known precipitate from a water soluble sample

Ammonia
Weak Base
Sulfuric Acid
Strong acid
Exp. 2 (gravimetric analysis)- What is the precipitate formed?
MgNH4PO46H2O
^finding this mass will help you find mass % of phosphorus

Exp. 2 (gravimetric analysis)- What substance should you use to rinse precipitation flask to ensure you have collected all of the solids in the funnel?
Acetone

Exp. 2 (gravimetric analysis)- Procedure
1. Form water soluble solution
- Get 1.0 g sample fertilizer
- Dissolve in 25 mL of water
- Stir and add activated carbon
2. Forming precipitate
- Use vacuum filtration to filter
- Add 15 mL of 0.5M MgSO4 (aq) solution
- Add 20 mL of ammonia (NH4OH)
3. Isolating precipitate
- Vacuum filtration to filer again
- Solid should be white
- Rinse flask with acetone to collect all remaining solid
- Measure and weigh
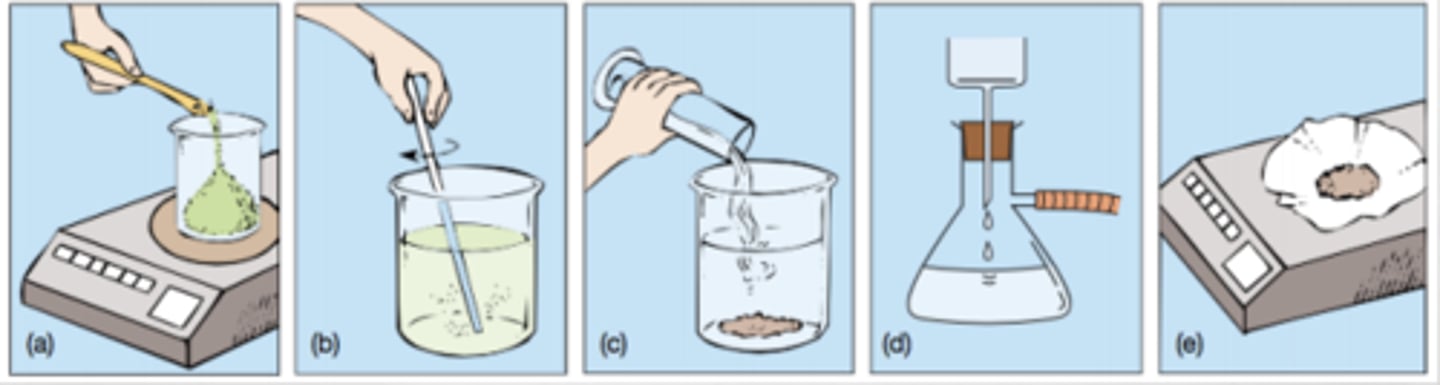
Exp. 2 (gravimetric analysis)- Calculations
1. Calculate the mass % phosphorus in the sample of plant food
2. Convert mass % phosphorus in your sample to mass % P2O5 in the sample
3. Find mass % P2O5 fertilizer content in the Miracle Gro sample
4. Calculate the percent error of mass % P2O5 compared to true value on fertilizer website
Exp. 2 (gravimetric analysis)- Discussion and error
Potential errors
- Potential loss of precipitate or increased mass due to impurities
- Solid was not properly filtered (too much solid left in the funnel)
- Not enough activated carbon was added, which removes copper cations from solution
- Water was used to wash sample instead of acetone, which would dissolve precipitate
Exp. 3 (copper cycle)- Objective
Performing a multistep process in which various forms of copper are oxidized, precipitated, dehydrated, dissolved, and reduced back to the starting species of metallic copper.
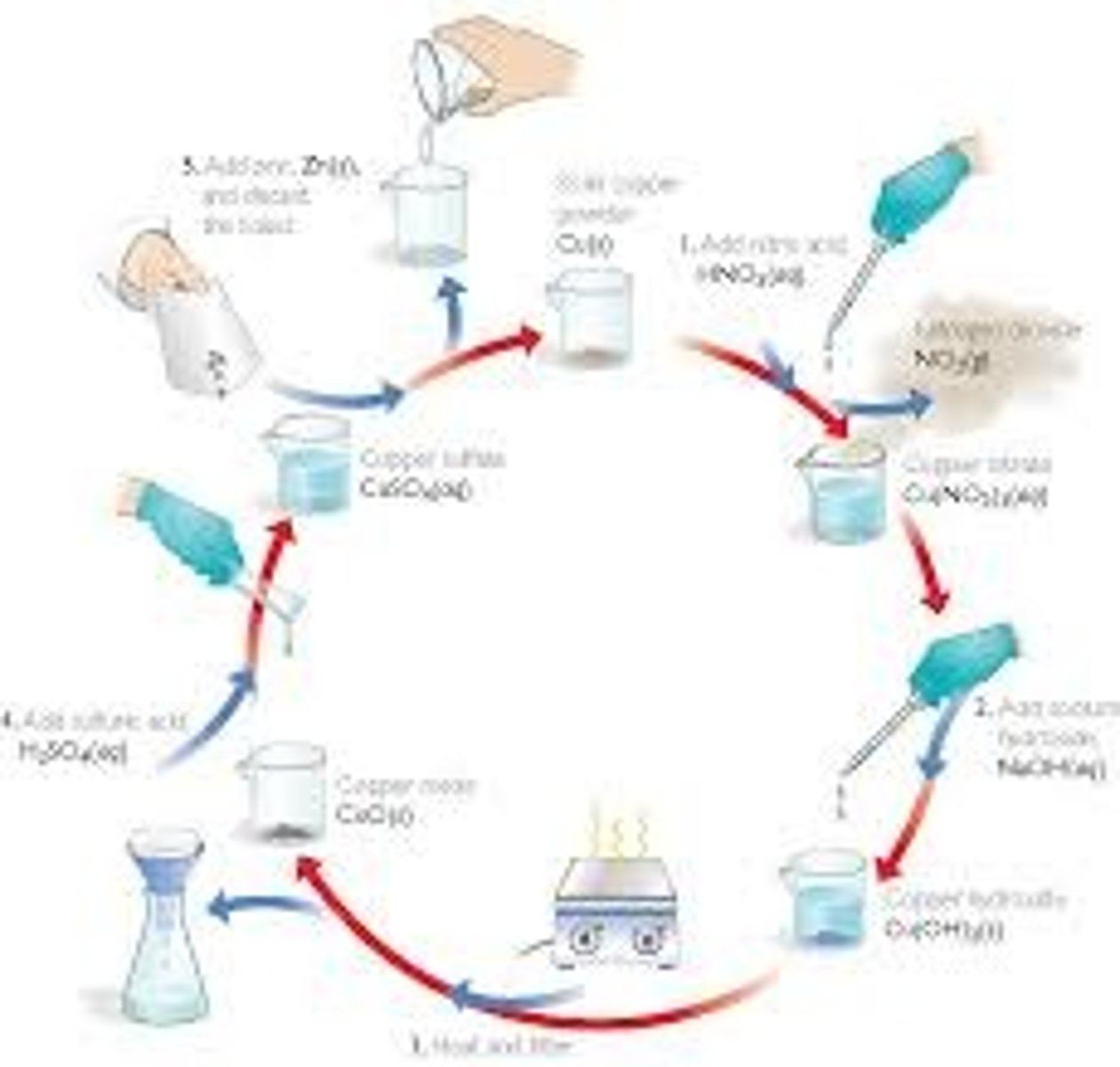
Exp. 3 (copper cycle)- Learning objectives
- Perform a series of chemical reactions
- Make qualitative observations about properties
- Using a bunsen burner properly
- Applying concept of percent yield
Exp. 3 (copper cycle)- When a solid metal is dissolving into a solution, is it being oxidized or reduced?
Oxidized
Exp. 3 (copper cycle)- Breakdown of copper cycle
1. Dissolution of copper metal
A solution with nitrate ions and hydrogens ions required.
2. Precipitation of copper hydroxide
Solution has copper now, so we need to separate it from the other components.
3. Dehydration of copper hydroxide
Dehydration reactions = product of water. Copper hydroxide is heated to form copper oxide and water .
4. Dissolution of copper oxide
Convert solid copper oxide to a soluble form. Solid copper oxide dissolves in acidic solutions.
5. Reduction of copper ions
Need a metal (Zinc) to reduce copper ions to copper metal
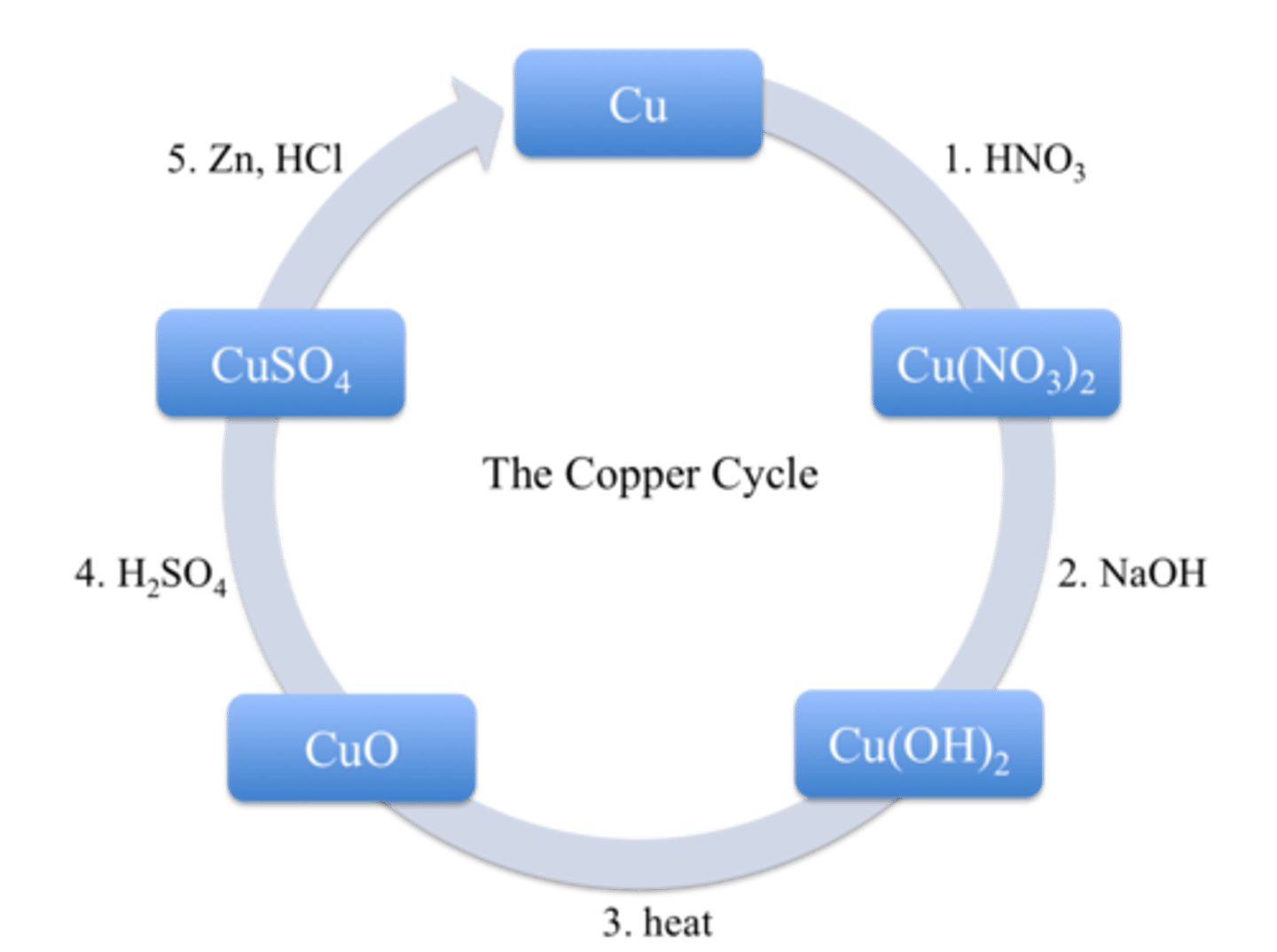
Exp. 3 (copper cycle)- Data
- Initial mass of copper metal recovered
- Final mass of copper metal recovered
- A LOT of qualitative observations
Exp. 3 (copper cycle)- Safety
Lab uses acids and bases at concentrations > 1M
ex. 16M nitric acid, so make sure your careful when handling +use gloves
Exp. 3 (copper cycle)- Procedure
1. Dissolution of copper metal
- Use 0.3- 0.4 gram copper metal sample
- 2 mL nitric acid
- Dilute w 10 mL DI water
2. Precipitation of copper hydroxide
- Add 1 mL of 5M NaOH
- Check solution with pH strip (ensure pH > 7)
3. Dehydration of copper hydroxide
- Add 75 mL of DI water
- Heat solution with bunsen burner for 5 minutes
- RINSE AND CLEAN FILTRATION FLASK so no residual sodium hydroxide is left over!
4. Dissolution of copper oxide
- Dissolve solid copper oxide in 10 mL of 3M sulfuric acid using vacuum filtration (make sure its turned off)
5. Reduction of copper ions
- Add 100 mg of powdered zinc to solution of copper ions
365 mg actually
- Wash and dry product to measure mass
Exp. 3 (copper cycle)- Calculations
Calculate percent yield
(Final mass of copper recovered / Initial mass of copper X 100%)

Exp. 3 (copper cycle)- Discussion and errors
Possible errors:
- Not enough NaOH was added (solution was not basic enough)
- Filtration flask was not cleaned well enough and residual sodium hydroxide was left over
- Dissolution part /vacuum filtration --> solid remains were left and was not filtered well enough
- Too much zinc was added
Exp. 3 (copper cycle)- What can you do if too much powdered zinc was added during the last step?
Add a few drops of hydrochloric acid
Exp. 9 (concentration of hydrogen peroxide)- Objective
Determine the concentration of a hydrogen peroxide solution volumetrically through an application of the ideal gas law to the volume of gas that is evolved when we decompose the hydrogen peroxide
(Find mass % concentration of H2O2 in a solution)

Volumetric analysis
Similar to gravimetric analysis, yet based on measurements of volume.
Involves the analysis of samples where reactants, products, or unreacted sample is in a gaseous form.

Exp. 9 (concentration of hydrogen peroxide)- Learning objectives
- Perform volumetric analysis of a gas evolution reaction
- Reaction catalyst and function in experiment
- Partial pressure
- Collecting gaseous sample over water
Exp. 9 (concentration of hydrogen peroxide)- How does hydrogen peroxide found in a drug store decompose normally>
3% Hydrogen peroxide decomposes VERY slowly
(around 1% per year)

Exp. 9 (concentration of hydrogen peroxide)- The decomposition of PURE liquid hydrogen peroxide is a highly __________ process?
EXOTHERMIC

Exp. 9 (concentration of hydrogen peroxide)- How can you speed the rate of reaction of hydrogen peroxide (when decomposing)?
Adding a reaction catalyst
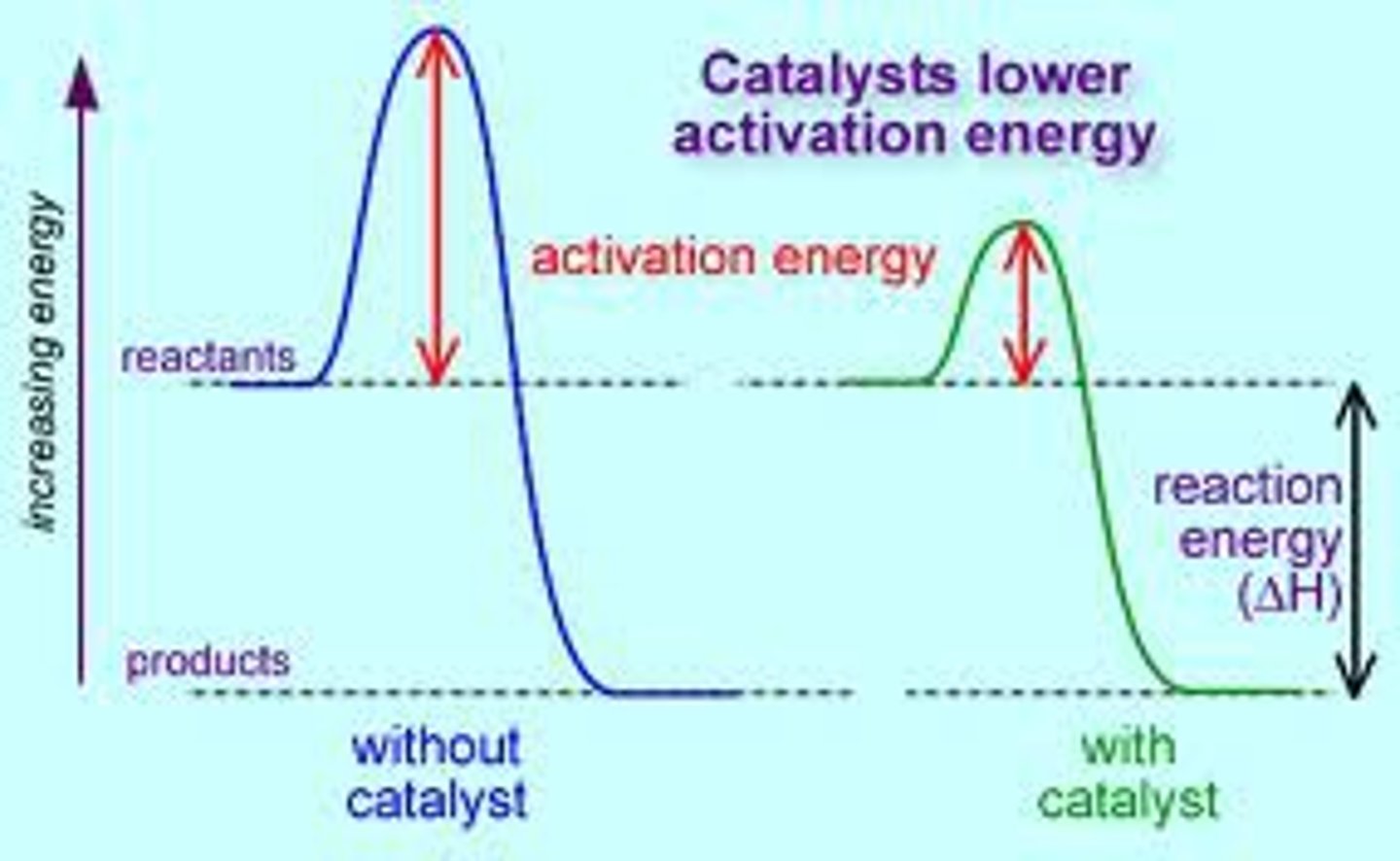
Reaction catalyst
A catalyst is a substance that speeds up a chemical reaction, but is not consumed by the reaction; hence a catalyst can be recovered chemically unchanged at the end of the reaction it has been used to speed up, or catalyze.
Exp. 9 (concentration of hydrogen peroxide)- What is the catalyst used in this experiment?
Potassium Iodide (KI)
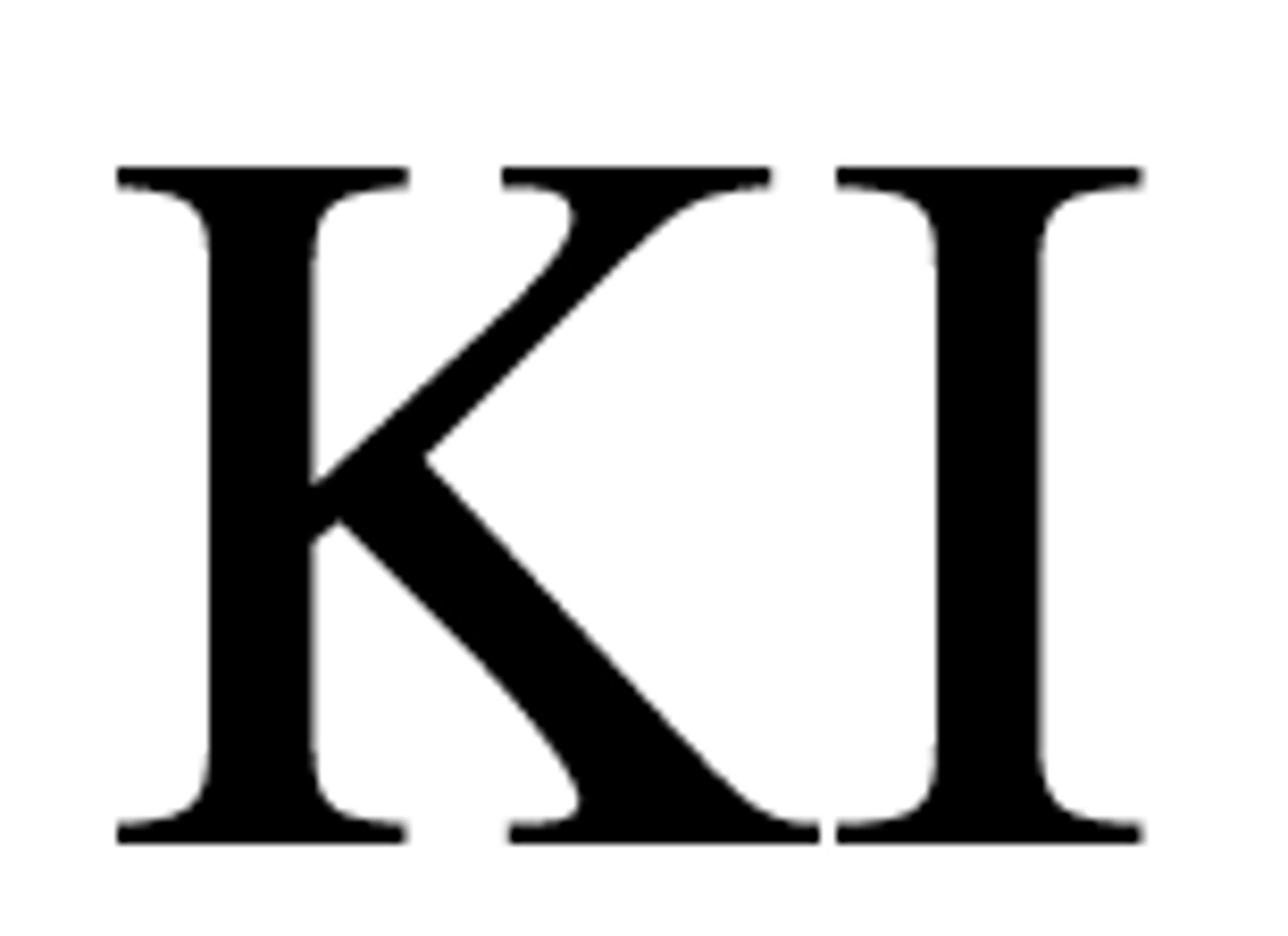
Exp. 9 (concentration of hydrogen peroxide)- Can hypoiodite (IO) be used as a catalyst in the decomposition of hydrogen peroxide?
Yes, IO- is an catalyst
Exp. 9 (concentration of hydrogen peroxide)- Description of iodide- catalyzed decomposition of hydrogen peroxide
Hydrogen peroxide decomposes to form WATER AND OXYGEN
*without the consumption of the catalyst iodide

Exp. 9 (concentration of hydrogen peroxide)- Procedure
1. Deliver MAXIMUM 5 ML of hydrogen peroxide accurately and precisely using a clean graduated pipet into flask
2. Ensure water levels in A and B are equal (record A- initial)
3. Add 1/2 gram KI and quickly close flask
4. Gas will begin to evolve, lower Pipet B to equalize pressures VISUALLY NOT NUMBERS
5. Gas stops, ensure pressures/water level are equalized
6. Record A again (final)
*repeat 3 times= 3 trials
Exp. 9 (concentration of hydrogen peroxide)- Is it necessary to deliver hydrogen peroxide accurately and precisely?
YES
5 mL of hydrogen peroxide BOTH accurately and precisely
Exp. 9 (concentration of hydrogen peroxide)- How many decomposition trials?
3 total
Exp. 9 (concentration of hydrogen peroxide)- Why was it necessary to equalize water levels inside the two pipets?
This would ensure that the total pressure inside Pipet A = atmospheric pressure in the lab
Exp. 9 (concentration of hydrogen peroxide)- Why is the change in volume reading of Pipet A so important?
Avogadro's Law:
Volume of oxygen gas produced IS PROPORTIONAL to Moles of oxygen gas produced
*this works because we kept the water levels equalized, ensuring that the pressure in A is equal to atmospheric pressure
Exp. 9 (concentration of hydrogen peroxide)- What data is needed from this lab?
- Volume of solution (hydrogen peroxide solution)
- Volume of gas produced (change in Pipet A)
- Barometric pressure (given in the room)
- Temperature of room
Exp. 9 (concentration of hydrogen peroxide)- Calculations
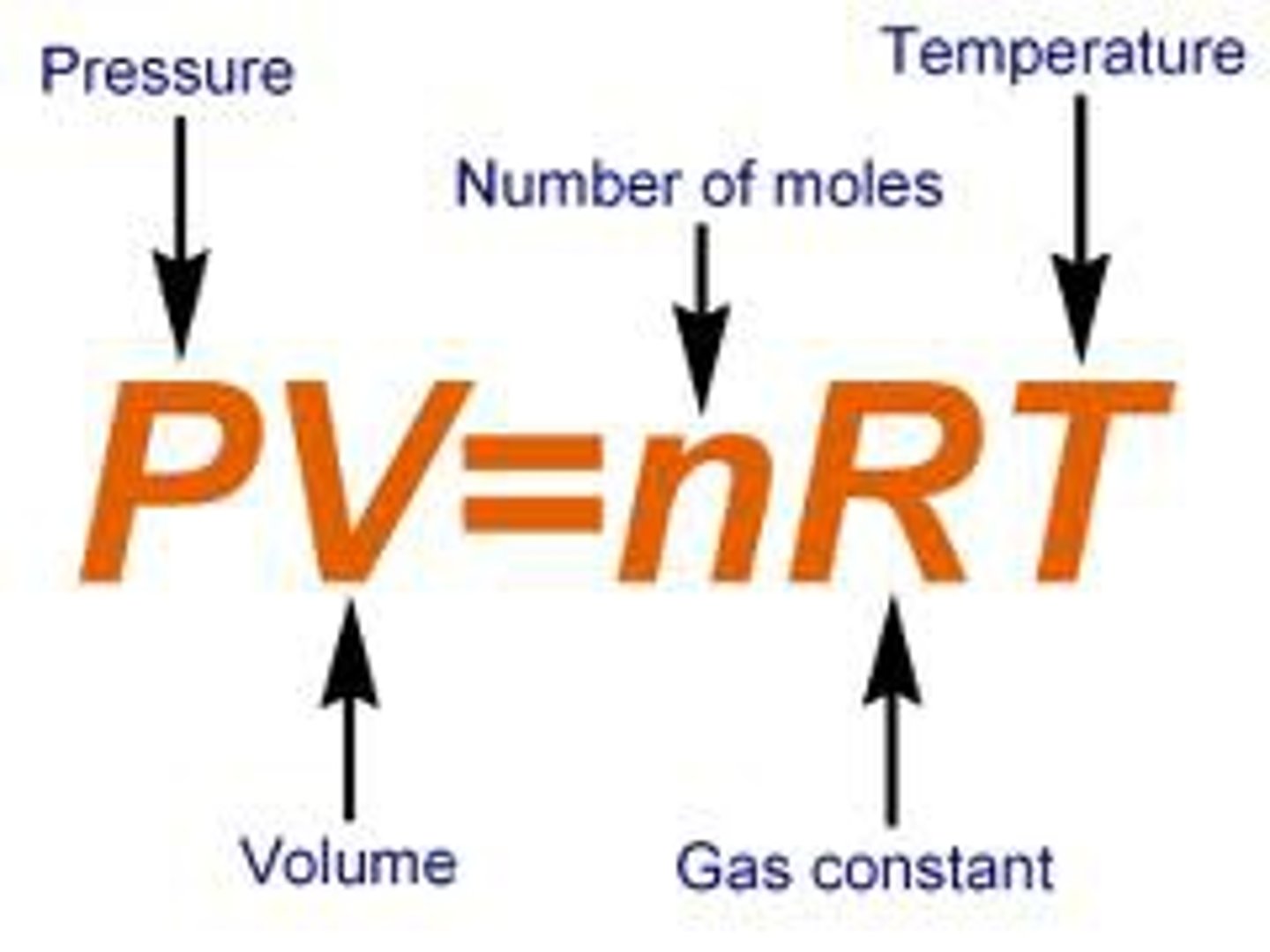
1. Find moles of oxygen produced (using ideal gas law)
2. Find number of moles of hydrogen peroxide present in the 5 mL sample
(FINAL) 3. Mass/ Volume % of hydrogen peroxide in sample + error
Exp. 9 (concentration of hydrogen peroxide)- Discussion and error
Possible errors:
- Not equalizing the water levels between pipets A and B, this would mean the total pressure in pipet A was NOT equal to the atmospheric pressure in the room (affects the mole calculation /Avogradro's law)
- Flask was not stopped quick enough (ex. more than 10 seconds passed), this would allow gas to ESCAPE and less gas would be collected)
Exp. 5 (calorimetry)- Objective
Raise temp of hand about 20 degrees celsius
Design a heat pack for maintenance workers on the Alaskan pipeline. Heat pack should be able to
Exp. 5 (calorimetry)- Learning objectives
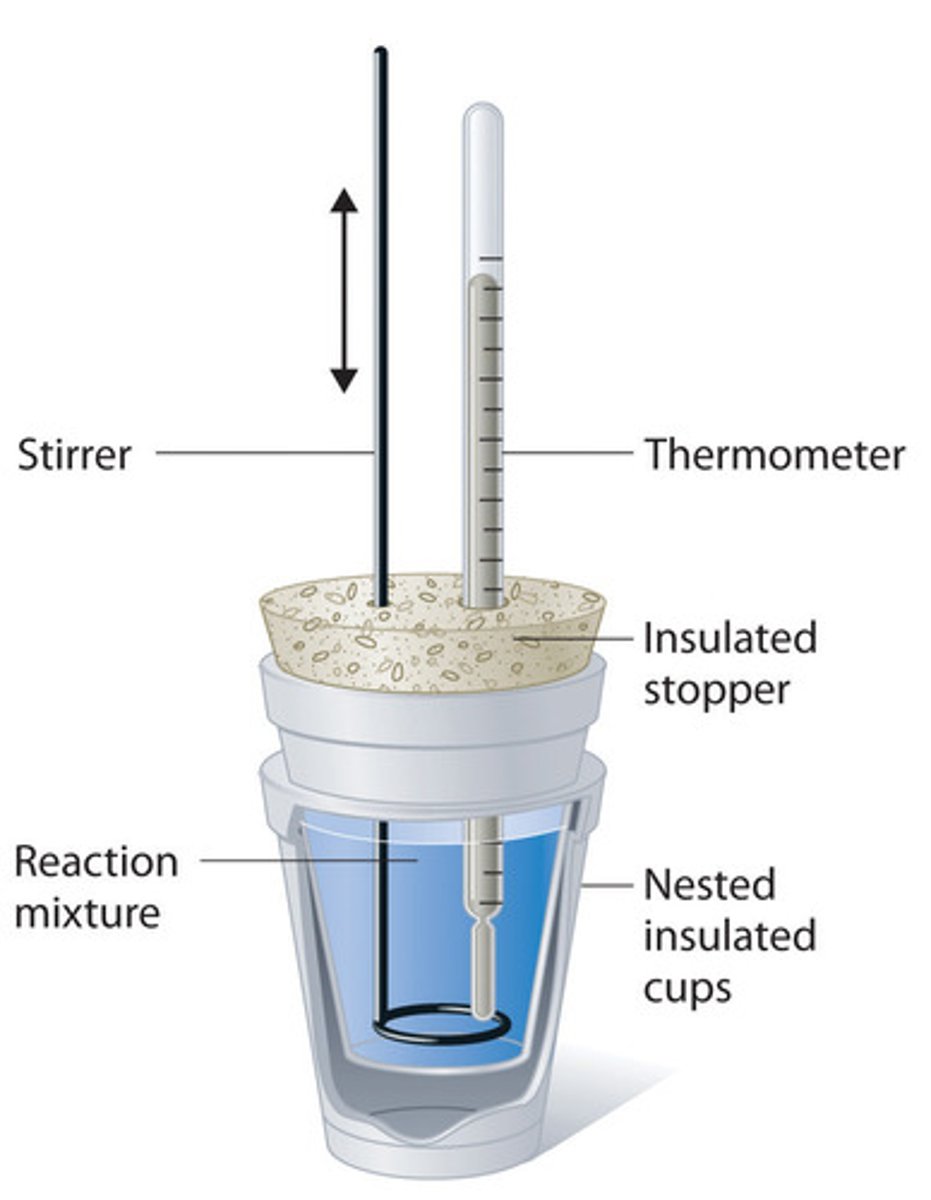
- Assemble a coffee cup calorimeter
- 1st Law of Thermodynamics
- Heat capacity of human hand
- Dissolve various salts in aqueous solution- endothermic or exothermic
- Enthalpy of reactions
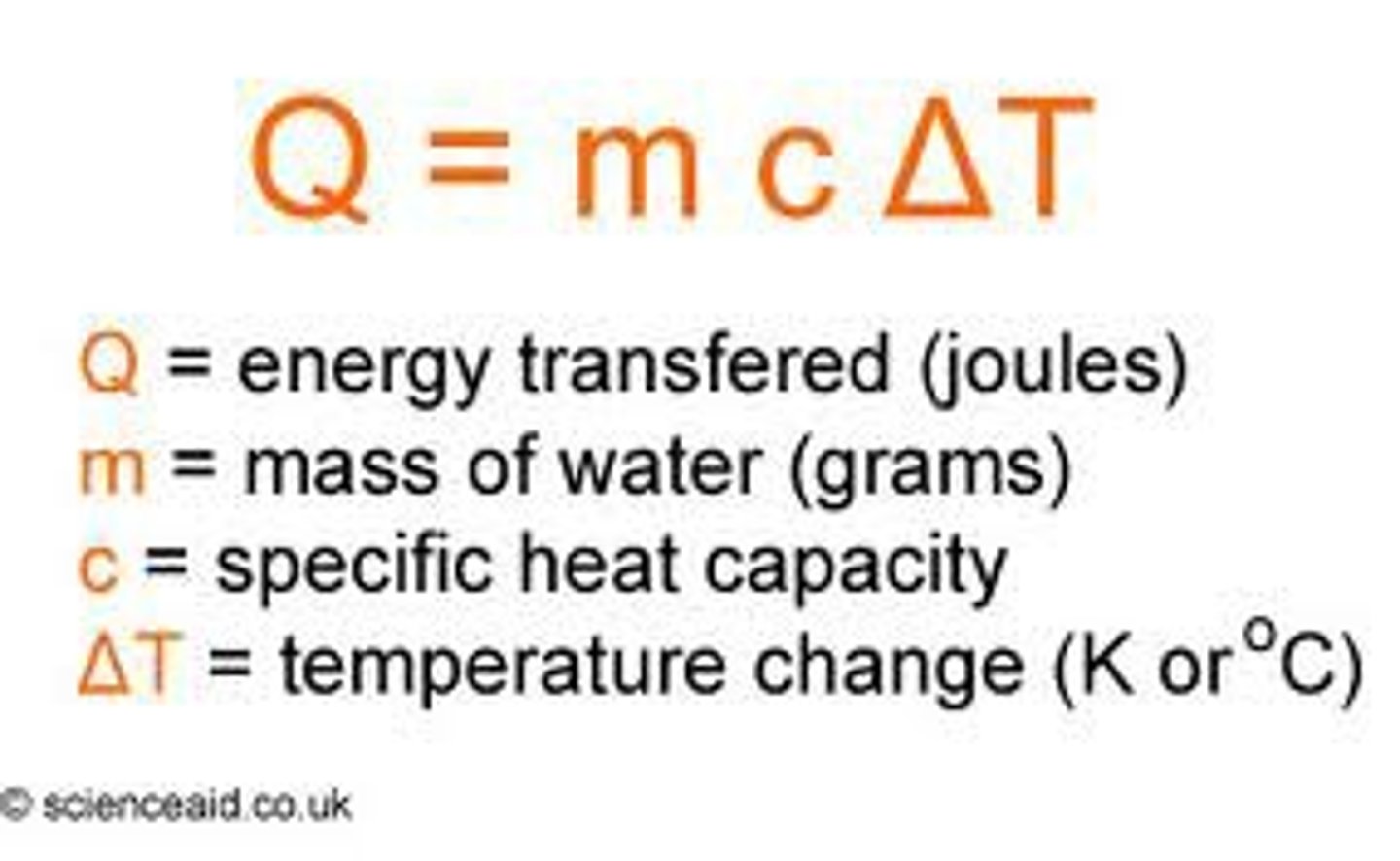
Specific Heat Capacity
The amount of ENERGY required to raise the temperature of ONE GRAM of a substance by ONE DEGREE CELSIUS
Exp. 5 (calorimetry)- What will be used in place to represent a human hand/ heat capacity of a human hand?
Vienna Sausages

Exp. 5 (calorimetry)- What is the use of the calorimeter?
To measure the specific heat capacity of Vienna sausages
To measure enthalpy of dissolution of salts in aqueous solutions
1st Law of Thermodynamics
The total amount of energy in the universe is constant
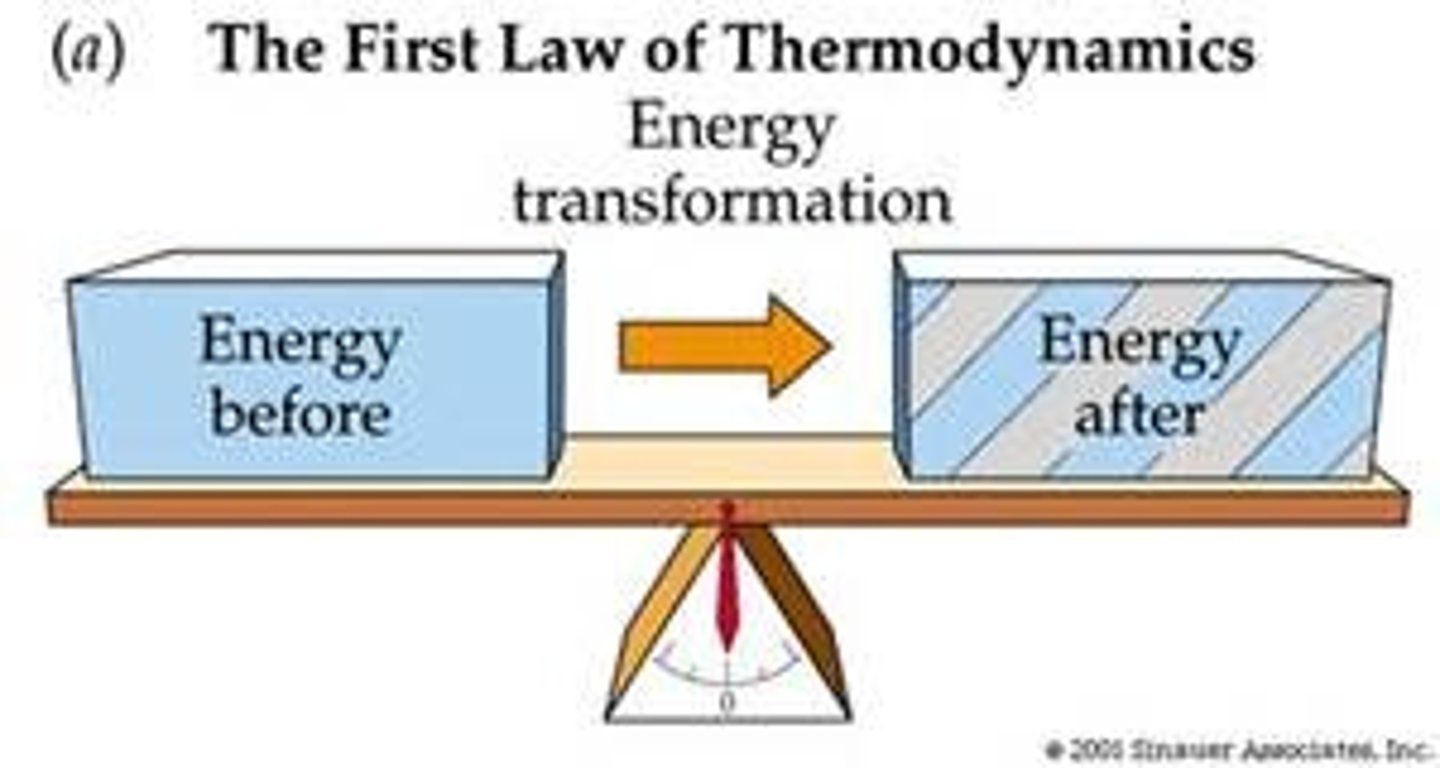
Enthaply change
The change in heat energy accompanying a chemical reaction

Endothermic
Reactions where heat is absorbed
Positive enthaply

Exothermic
Reactions where heat is released
Negative enthalpy

Exp. 5 (calorimetry)- What type of system is the calorimeter?
Adiabatic system
Adiabatic system
A system in which heat is not allowed in or out
ex. calorimeter or thermos bottle

Closed system
System where heat may be transferred, but not mass.
ex. a hot water bottle

Open system
System where both heat (energy) and mass may be transferred

Exp. 5 (calorimetry)- Procedure
Finding heat capacity of hand-
1. Create ice slush
2. Get 3 sausages and place into slush for 10-15 min
3. Assemble calorimeter w 100 mL of DI water
4. Find mass of water and sausage using "by difference"
5. Get initial and final temp.
Picking salt to use:
1. Find which salt to use
2. Get 2 mL of DI water and pour 0.20 grams of salt
3. Pick salt that is exothermic ( temp. of calorimeter will increase)
Measure enthalpy (heat produced by salt dissolution):
1. 60 mLof DI water in calorimeter
2. Add 4.0 grams of salt
3. Measure initial and final temp after a minute
*3 total salt trials, 60 mL, 40 mL, 20 mL
Exp. 5 (calorimetry)- Why did you have to estimate how many sausages fit on to an average human hand?
To determine the average heat capacity of a human hand
Exp. 5 (calorimetry)- Calculations
1. Calculate mass of average hand
2. Calculate specific heat capacity of sausage
3. Calculate specific heat capacity of average hand
4. Report salt- Anhydrous Magnesium sulfate MgSO4 (exothermic)
5. Calculate molar enthalpy of dissolving salt
Exp. 5 (calorimetry)- Discussion and errors
- Chose the wrong salt, one that is endothermic
- Measured final temperature when they were not constant yet, may not have been accurate
Exp. 6 (flame test)- Objective
Understand the interaction between light and matter. Understand why ionic solutions produce colored flames.

Exp. 6 (flame test)- Learning objectives
-Observe colors of flames produced by aqueous ionic solutions
- Why ionic solutions produce colored flames
- Identify composition of unknown ionic solutions
Spectrometer
A device that spreads light into its different colors
Is blue light more energetic than red light?
True, because wavelength is smaller
Light rays with lower energy have smaller wavelengths?
False
Smaller wavelength = Higher energy
Longer wavelength = Lower energy
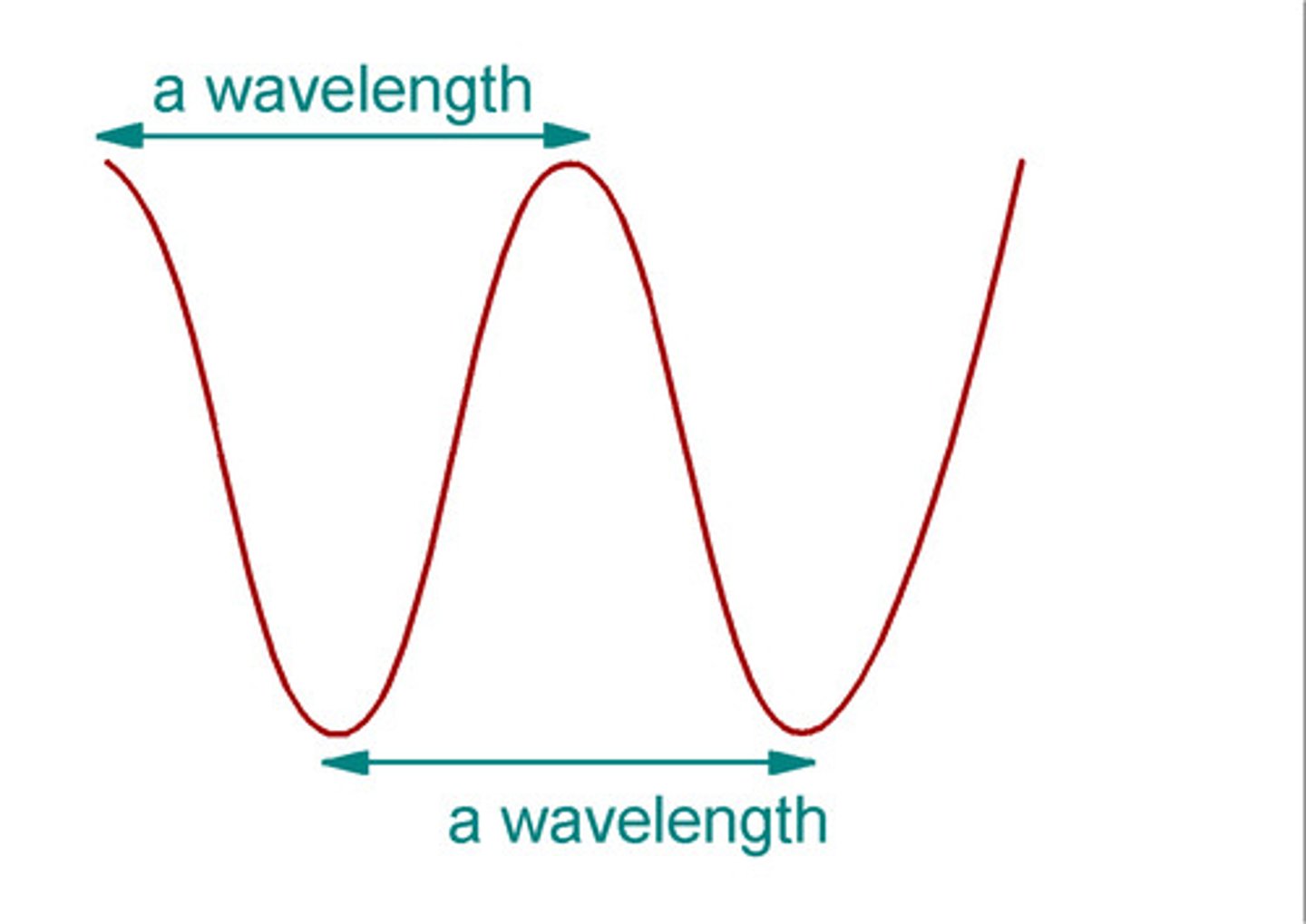
Exp. 6 (flame test)- Why is the color of the flame so important?
Light/ color emitted can be used to signal the presence of a PARTICULAR element
Exp. 6 (flame test)- How do ionic solution flames produce color?
Ionic solutions are EXCITED (by adding heat)
When atoms are excited to a higher energy state, they EMIT characteristic color
Exp. 6 (flame test)- What is the purpose of heating nichrome (nickel-chromium) in flame?
To atomize (break down to atoms) ionic solution into fine droplets in the air vent of bunsen burner.

Exp. 6 (flame test)- Where is the hottest part of the flame?
The tip of the inner blue flame
Exp. 6 (flame test)- Procedure
Perform on series of known and.1 unknown solution
1. Clean loop of nichrome wire in 1M HCl + then water + then burner flame
2. Place drops of solution to watch glass
3. Place watch glass near air vent
4. Heat nichrome in flame + then onto the watch glass
5. Observe quick color change of flame

Exp. 6 (flame test)- Do you have to clean nichrome wire in between trials
YES
always clean wire in between trials
Exp. 6 (flame test)- Calculations
Known solutions:
Group solutions based on the color they emitted (similarity is the ion they share)
Unknown solution:
Guess what ion it must contain based on the color it produced
Exp. 6 (flame test)- Discussion and errors
- Did not clean nichrome wire per trial
- Did not heat nichrome wire enough to notice a color change in the flame
Exp. 6 (flame test)- Are flame tests best for testing the presence of cations or anions?
Cations
That was the ion each solution had in common, not their anions

Exp. 7 (spectroscopy)- Objective
Gain an introduction to visual spectroscopy
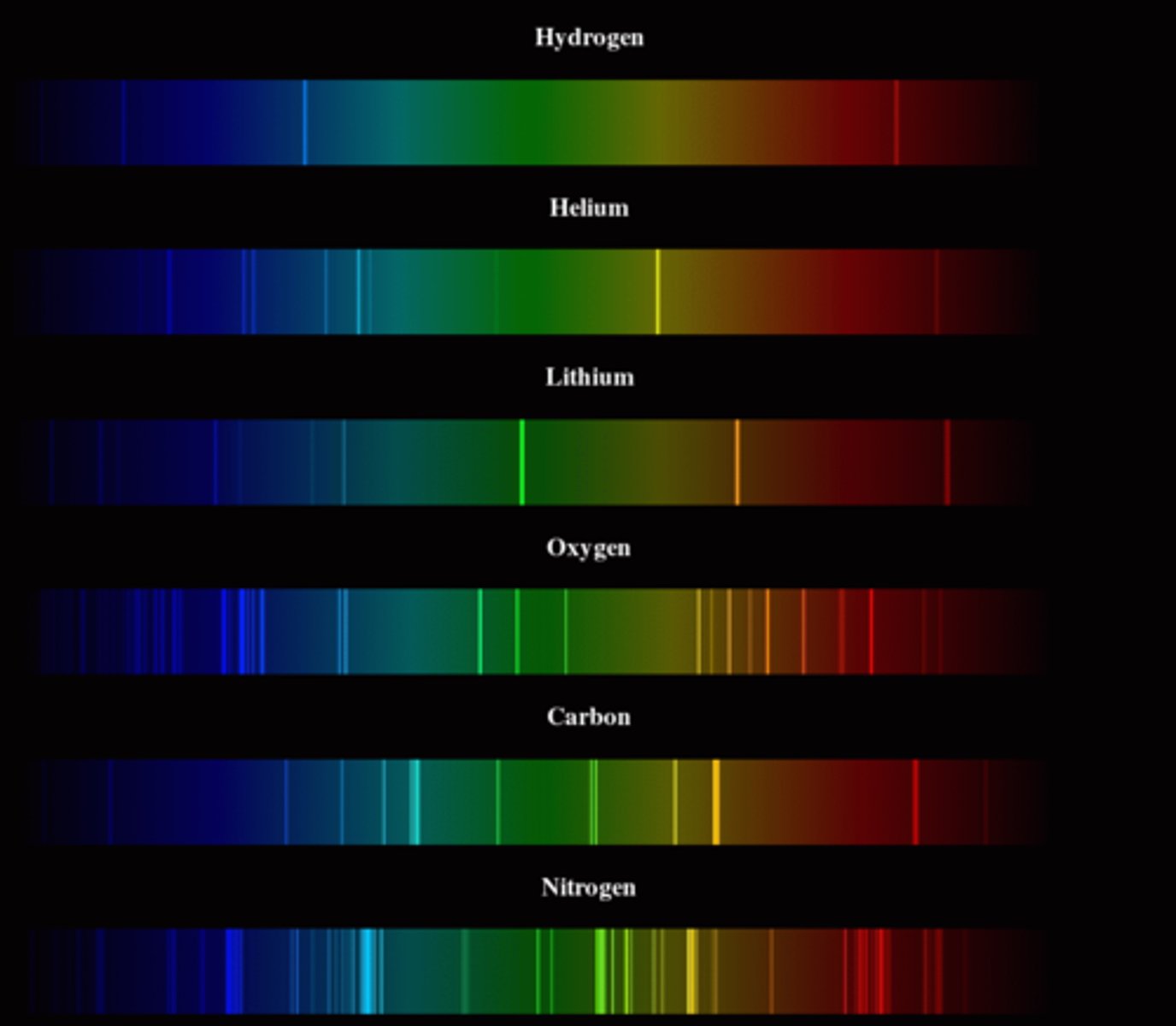
Exp. 7 (spectroscopy)- Learning Objectives
-Using visible- near IR spectrometers to measure absorption spectra
- Understand purpose and composition of "blank" solution
- Wavelength maximum of a spectrum
- Relationship between absorbance of light, chemical nature, path length, analyte concentration
Spectroscopy
The study of the interaction between light and matter
Absorption spectrum
The range of a pigment's ability to absorb various wavelengths of light.
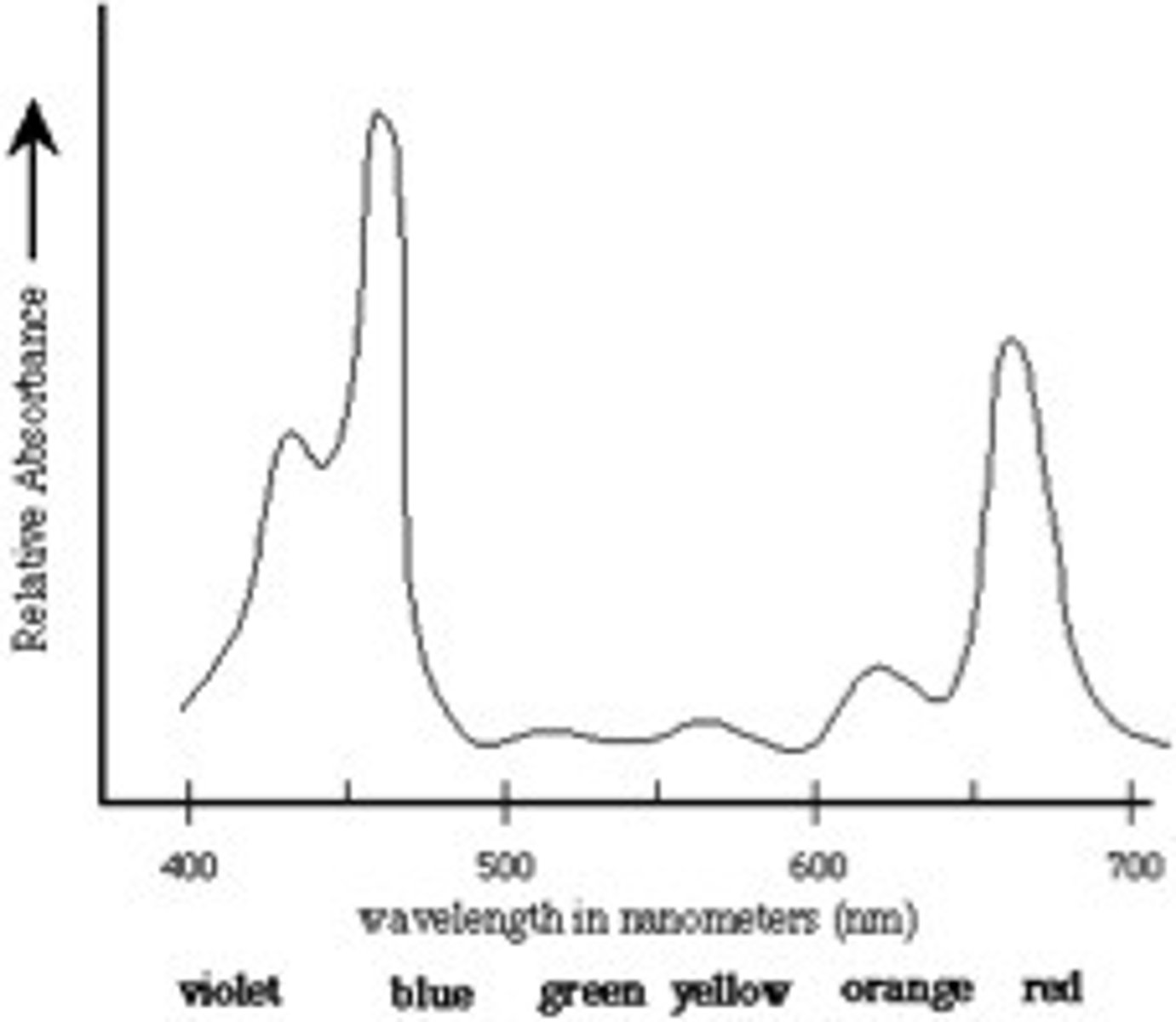
Wavelength max
Wavelength in the spectrum where the absorbance reaches a max

Exp. 7 (spectroscopy)- Can molecules absorb light of specific wavelengths or frequencies?
Yes
Exp. 7 (spectroscopy)- Ca and Ca 2+ will have different absorption spectra?
True, they would have different spectra
Exp. 7 (spectroscopy)- When a molecule returns down to its ground state, what does it release?
A photon
Exp. 7 (spectroscopy)- Light has both wavelength and particle like properties
True
Exp. 7 (spectroscopy)- What are some materials cuvettes can be made of?
Glass, Quartz, Plastics

Spectrophotometry
Measurements of the number of photons of light emitted or absorbed that can be used for quantitative analysis
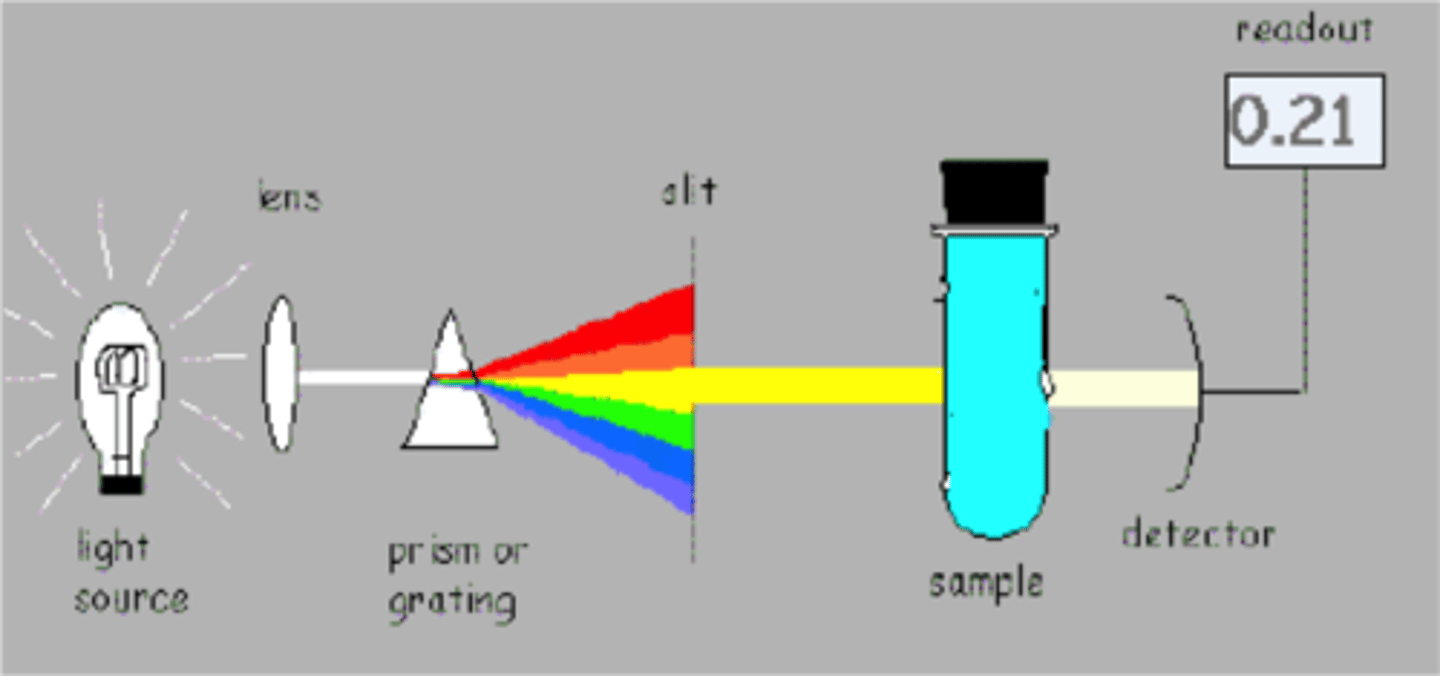
Vernier Visible- Near IR Spectrophotometer
Range from 380- 900 nm