First Law and Energy Conversion
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
13 Terms
first law of thermodynamics
for all adiabatic processes between two specified states of a closed system, the net work done is the same regardless of the nature of the closed system and the details of the process
energy balanace
energy in - energy out = total change in energy of the system
energy change of a system
total change in energy = final energy - initial energy
= change in internal energy + change in kinetic energy + change in potential energy
is energy a property
yes, therefore the energy change is zero if the state of the system does not change
mechanisms of energy transfer
heat transfer, work transfer, mass flow (unless it is a closed mass system)
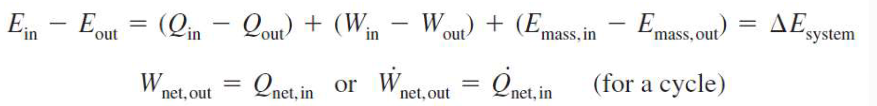
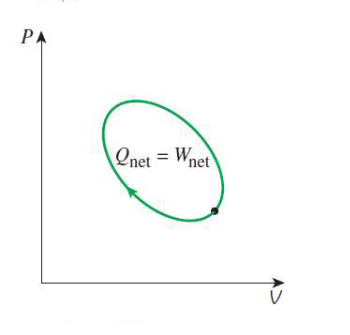
change in energy for a cycle
change in energy = 0
therefore Q = W
efficiency
indicates how well and energy conversion or transfer process is accomplished
efficiency = desired output/required input
heating value of fuel (HV)
the amount of heat released when a unit amount of fuel at room temperature is completely burned and the combustion products are cooled to the room temperature
generator
a device that converts mechanical energy to electrical energy
generator efficiency
the ration of the electrical power output to the mechanical power output
thermal efficiency of a power plant
the ratio of the net mechanical output to the rate of heat input
products of the combustion of fuel
carbon dioxide, nitrogen oxides, carbon monoxide, sulfur dioxide
benefits of energy-efficient appliances
helps the environment by reducing the amount of pollutants emitted to the atmosphere during the combustion of fuel