group 7- the halogens
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
16 Terms
describe the appearance of these halogens
at room temperature and pressure
in aqueous solutions
F2,CL2, Br2, I2
F2
-room: Yellow gas
-aq: colourless solution
CL2-
-room: pale green gas
-aq: very pale green solution/ colourless solution
Br2-
-room: brown-red liquid
-aq: orange solution
I2-
-room: black/grey shiny solid (purple vapour)
-aq: brown solution
describe the atomic radius down group 7
increases
addition of new electron shells, places outer electrons further from the nucleus,
new inner shells cause increased shielding of outer electrons from the positive nuclear charge,
weakening the overall nuclear attraction despite more protons.
describe the trend in electronegativity down group 7
decreases
atomic radius increases
shielding increases
weaker attraction between nucleus and electrons in covalent bond
describe the trend in boiling point down group 7
boiling point increases
the Mr increases down the group
the strength of VDW’s forces between molecules increases
the energy needed to overcome IMF’s increases
state and explain the trend in oxidising ability of the halogens down group 7
decreases
chlorine can oxidise Br- to Br2
chlorine can oxidise I- to I2
bromine can oxidise I- to I2
what is an alternative name for the experiment that demonstrates oxidising ability of halogens
displacement
what colour are these aq halide salt solutions
KCl
KBr
KI
they are colourless
what colour is a solution of-
Cl2
Br2
I2
Cl2 - colourless/ pale green solution
Br2 - orange solution
I2 - brown solution
colour changes for displacement reactions of KCl, KBr and KI with Cl2, Br2 and I2
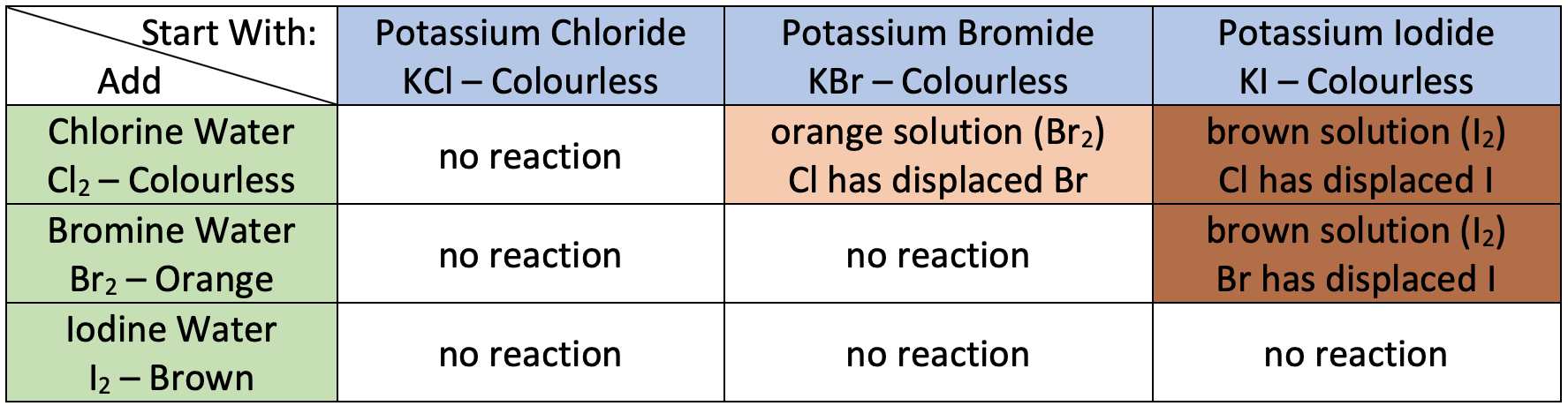
describe in words the reactions without colour changes In terms of oxidising ability of halogens
bromine is not a strong enough oxidising agent to displace chlorine from potassium chloride (OR bromine from potassium bromide)
iodine is not a strong enough oxidising agent o displace *halogen* from potassium chloride/bromide/iodide
chlorine is not a strong enough oxidising agent to displace chlorine from potassium chloride
give the balanced equations for displacement reactions of aq halide ions
identify product responsible for colour change
Cl2 + 2KBr → 2KCl + Br2
Cl2 + 2KI → 2KCl + I2
Br2 + 2KI → 2KBr + I2
give the ionic equations for the reactions that give colour change in halogen displacement reactions (remove spectator ions)
Cl2 + 2Br- → 2Cl + Br2
Cl2 + 2I- → 2Cl + I2
Br2 + 2I- → 2Br + I2
describe in words the reactions with colour changes In terms of oxidising ability of halogens
chlorine is a strong enough oxidising agent to displace bromine from potassium bromide
chlorine is a strong enough oxidising agent to displace iodine from potassium iodide
bromine is a strong enough oxidising agent to displace iodine from potassium iodide
why is chlorine a good oxidising agent
it is readily reduced due to its high electronegativity (little shielding, small atomic radius so strong nuclear charge)
so it can easily accept an electron from another molecule causing it to be oxidised
describe the reaction that demonstrates the reducing ability
the reactions of solid sodium halides ( NaCl, NaBr, NaI) with concentrated sulphuric acid
state and explain the reducing ability of halide ions down the group
increases
down the group ionic radius increases
so the outer electrons have weaker attraction to the nucleus so are more easily lost