MCAT Biochemistry Chapter 11- Lipid and Amino Acid Metabolism
1/86
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
87 Terms
dietary fats consist mainly of ____.. but also what?
triacylglycerol (primary),
also cholesterol, cholesteryl esters, phopholipids, and free fatty acids
Where does most lipid digestion occurs?
Small intestine (duodenum = where emulsification occurs)
Emulsification
-the mixing of two normally immiscible liquids (in this case fat and water)
-formation of an emulsion = increases the surface are of the lipid, which permits greater enzymatic interaction and processing
-occurs upon entry into the duodenum
-aided by bile
Bile contains...
-bile salts
-pigments
-cholesterol
Bile is secreted by the... and stored in the ...
-liver
-gallbladder
Enzymes secreted by the pancreas
-pancreatic lipase
-colipase
-cholesterol esterase
Pancreatic enzymes hydrolyze lipid components to...
-2-monoacylglycerol
-free fatty acids
-cholesterol
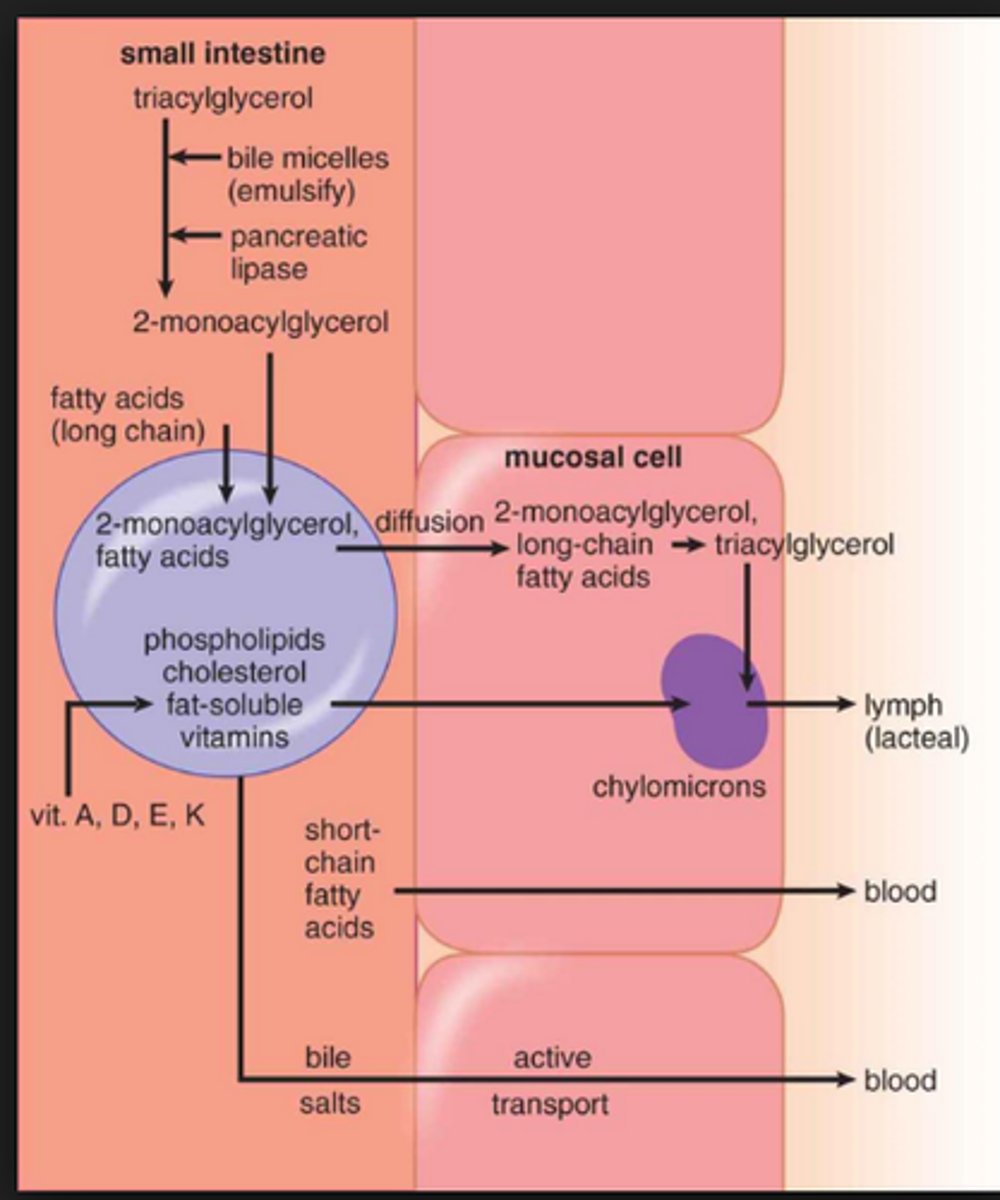
emulsion is followed by ____
absorption of fats by intestinal cells.
free fatty acids, cholesterol, 2-monoacylglycerol, and bile salts contribute to the formation of micelles
Micelles
-clusters of amphipathic lipids that are soluble in the aqueous environment of the intestinal lumen
-free fatty acids, cholesterol, 2-monoacylglycerol, and bile salts contribute to their formation
-vital for digestion, transport, absorption of lipid soluble substances starting from dodenum to ileum
at the end of the ileum what happens?
bile salts are actively reabsorbed and recycled;
any fat that remains in the intestine will pass into the colon and end up in stool.
Micelles difusse to the...
brush border of the intestinal mucosal cells where they are absorbed. the digested lipids pass thru the brush border where they are absorbed into mucosa and re-esterified to form triacylglycerols and cholesteryl esters
-they are packaged, along w/ apoproteins, fat soluble vitamins and other lipids, into chylomicrons
Lacteals
vessels of the lymphatic system. chylomicrons leave the intestine via lacteals and re-enter the bloodstream via the thoracic duct
Thoracic duct
-long lymphatic vessel that empties into left subclavian vein at the base of the neck
-responsible for the re-entry of chlyomicrons into the bloodstream
Short-chain fatty acids are absorbed...
across the intestine via simple diffusion directly into the blood
Long-chain fatty acids are absorbed...
as micelles and assembled into chylomicrons for release into the lymphatic system
Post absorptive state
at night body is in postabsorptive state, utilizing energy stores instead of food for fuel
fatty acids are released from adipose tissue and used for energy
Hormone-sensitive lipase
-hydrolyzes triacylglycerols yielding fatty acids and glycerol
-activated by epinephrine, cortisol, and a fall in insulin levels
-released glycerol may be transported to the liver for glycolysis or gluconeogenesis
-doesn't respond to glucagon
-effective in adipose cells but need LPL for metaboism of chylomicrons and VLDL
Lipoprotein lipase (LPL)
-necessary for metabolism of chylomirons and very-low-density lipoproteins (VLDL)
-can release free fatty acids from triacylglycerols in these lipoproteins
free fatty acids are transported thru blood in association w/ _____
albumin, a carrier protein
Lipoproteins
-aggregates of apolipoproteins and lipids
-how triacyl glycerol and cholesterol are transported in blood
-named according to their density, which increases in direct proportion to the percentage of protein in the particle
Chylomicrons
-least dense lipoproteins (highest fat:protein ratio)
-highly soluble in both lymphatic fluid and blood
-function in transport of dietary triacylglycerol, cholesterol, and cholesteryl esters to other tissues
-assembled in the intestinal lining = results in nascent chylomicrons that contain lipids and apolipoproteins
summary of lipoprotein classes
Chylomicrons and VLDL primarily carry triacylglycerols but also small quantities of cholesteryl esters
LDL and HDL primarily cholesterol transport
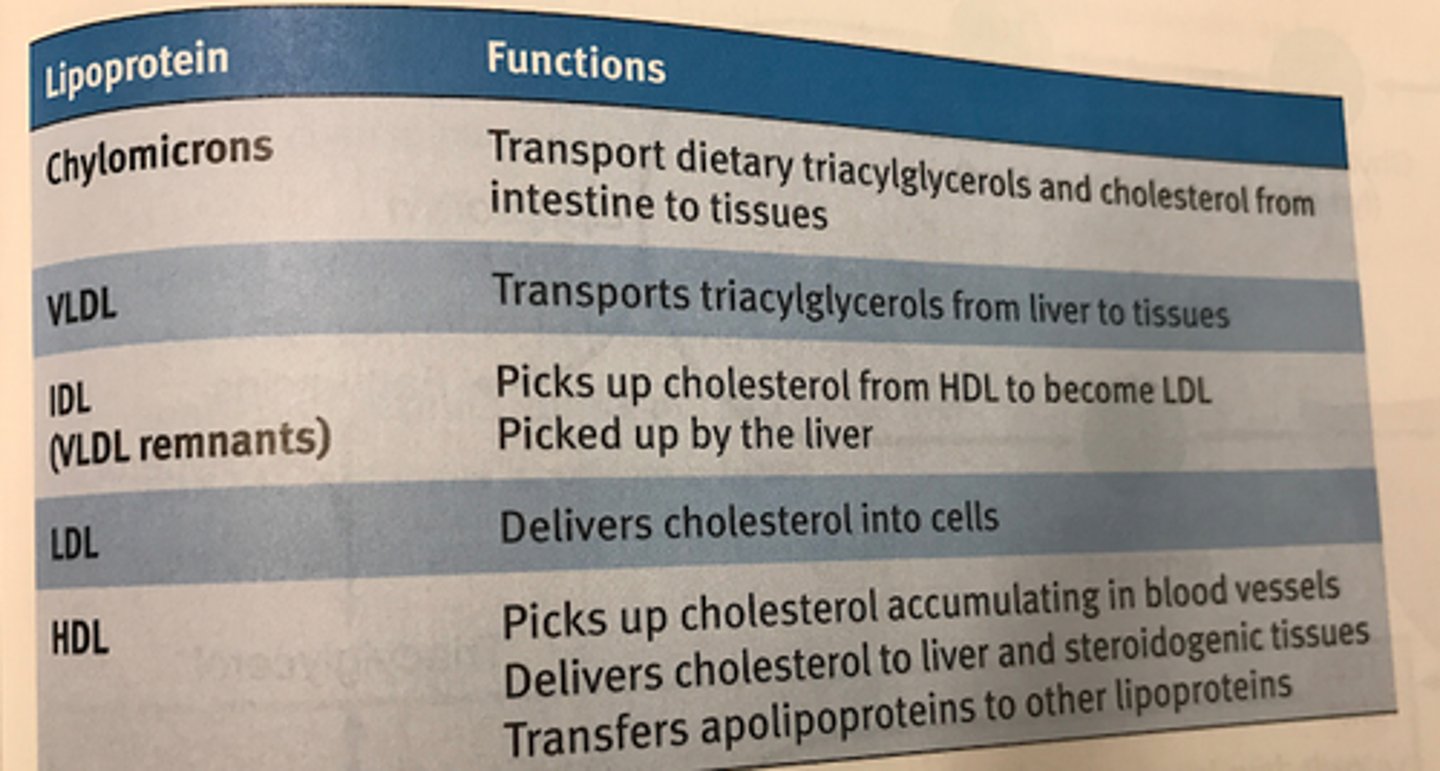
VLDL
-very-low-density lipoprotein
-produced and assembled in liver cells
-transport triacylglycerols from liver to tissues
-contain fatty acids that are synthesized from excess glucose or retrieved from chylomicron remnants
IDL
-intermediate-density lipoprotein, aka VLDL remnant
-transition between triacylglycerol and cholesterol transport
-some IDL = reabsorbed up by the liver by apolipoproteins on its exterior, some is further processed in the bloodstream
example of IDL being processed in bloodstream?
some IDL picks up cholesteryl esters from HDL to become LDL.
so is transition particle btwn triacylglycerol transport and cholesterol transport
LDL
-low-density lipoprotein
-primarily transports cholesterol for use by tissues in biosynthesis and cell membrane maintenance
roles of cholesterol
biosynthesis, cell memrbane stability/fluidity, bile acids and salts are made from cholesterol in the liver
many tissues require cholesterol for steroid hormon synthesis (steroidogenesis)
HDL
-high-density lipoprotein
-synthesized in liver and intestines; released as dense, protein rich particles in the blood.
-contain apolipoproteins for cholesterol recovery
-main functions is cholesterol recovery: cleaning up of excess cholesterol from blood vessels for excretion
-also delivers some cholesterol to steroidogenic tissues
Apolipoproteins
-aka apoproteins
-form the protein component of lipoproteins
-are receptor molecules involved in signaling
-control interactions between lipoproteins
brief summary of apolipoproteins (don't need to know for MCAT
apoA-I: activates LCAT, an enzyme that catalyzes cholesterol esterification
apoB-48: mediates chylomicron secretion
apoB-100: permits uptake of LDL by liver
etc
Cholesterol
-ubiquitous component in human body
-plays major role in synthesis of cell membranes, steroid hormones, bile acids, bile salts and vitamin D
sources of cholesterol
may be obtained through dietary sources (LDL or HDL)
or through de novo synthesis in the liver (driven by acetyl-CoA and ATP)
Citrate shuttle
carries mitochondrial acetyl-CoA into the cytoplasm, where cholesterol synthesis occurs
NADPH (from the pentose phosphate pathway) supplies reducing equivalents
rate-limiting step of cholesterol biosynthesis?
synthesis of mevalonic acid in the smooth ER.
-catalyzed by 3-hydroxy-3-methylglutaryl (HMG) CoA reductase
Regulation of cholesterol syntehsis
-inhibited by increased cholesterol (feedback inhibition)
-stimulated by insulin
-also dependent on HMG-CoA reductase gene expression in the cell
CETP
-cholesteryl ester transfer protein
-catalyzes the transition from IDL to LDL by transferring cholesteryl esters from HDL
LCAT
-lecithin-cholesterol acyltransferase
-enzyme found in bloodstream that is activated by HDL apoproteins
-adds a fatty acid to cholesterol to produce soluble cholesteryl esters (present in HDL... HDL cholesteryl esters can then be distributed to IDLs which become LDLs by acquiring these cholesteryl esters)
Nomenclature rules for fatty acids
-total number of carbons is given along with number of double bonds, written as carbons:double bonds
-further description can be given by indicating position and isomerism (cis or trans) of the double bonds in unsaturated fatty acid
Fatty acids
-long-chain carboxylic acids
-carboxyl carbon is carbon 1
-carbon 2 is referred to as alpha-carbon
-occur as salts that are capable of forming micelles or are esterified to other compounds
Omega ( ω) numbering system
-used for unsaturated fatty acids
- ω describes the position of the last double bond relative to the end of the chain and identifies the major precursor fatty acid
Two important essential fatty acids
-alpha-linolenic acid
-linoleic acid
-important for maintaining cell membrane fluidity
-humans can only synthesize a few unsaturated fatty acids, so the rest come from diet
Nontemplate synthesis
-synthesis that do not rely directly on the coding of a nucleic acid
-carbohydrate and lipid synthesis are most common examples
What are some things we can derive fatty acids from?
excess carbs and proteins from diet can be converted to fatty acids and stored as energy reserves in the form of triacylglycerol
major enzymes of fatty acid synthesis?
acetyl CoA carboxylase
fatty acid synthase
Fatty acid synthesis
-occurs primarily in liver and products are transported to adipose tissue for storage (adipose tissue can also synthesize smaller quantities of fatty acids
-synthesis occurs in the cytoplasm from acetyl-CoA transported out of the mitochondria
-includes five steps: activation, bond formation, reduction, dehydration, and second reduction
-repeated eight times to form palmitic acid (only fatty acid humans can synthesize)
Citrate lyase
splits citrate back into acetyl-CoA and OAA in the cytoplasm
Acetyl-CoA shuttling
-after large meal, acetyl-CoA accumulates in mitochondrial matrix and needs to be moved to cytosol for fatty acid biosynthesis
-acetyl-CoA = product of the pyruvate dehydrogenase complex and couples with oxaloacetate to form citrate (TCA)
-isocitrate dehydrogenase is the rate-limiting enzyme of citric acid cycle; as cell becomes energetically satisfied, it slows the citric acid cycle which causes citrate accumulation
-citrate diffuses across the mitochondrial membrane
-in cytosol, citrate lyase splits citrate back into acetyl-CoA and OAA
-OAA returns to mitochondrion to continue moving acetyl-CoA
Acetyl-CoA carboxylase
-rate-limiting enzyme of fatty acid synthesis
-activates acetyl CoA in the cytoplasm
-requires biotin and ATP to function
-adds CO2 to acetyl-CoA to form malonyl-CoA
-activated by insulin and citrate
Fatty acid synthase
-aka palmitate synthase
-large multienzyme complex in cytosol containing acyl carrier protein (ACP)
-ACP requires pantothenic acid (vitamin B5)
-also requires NADPH to reduce the acetyl groups added to the fatty acid
When is fatty acid synthase/palmitate synthase induced?
in the liver folowing a meal high in carbs bc of elevated insulin levels
steps of fatty acid synthase
-performs the following steps:
1. activation of growing chain and malonyl-CoA with ACP
2. bond formation between these activated molecules (releases CoA and CO2)
3. reduction of a carbonyl to a hydroxyl group (req. NADPH)
4. Dehydration (releases H2O)
5. Reduction to a saturated fatty acid (req. NADPH)
REPEAT again and again until the molecule you want (ie palmitate) is made
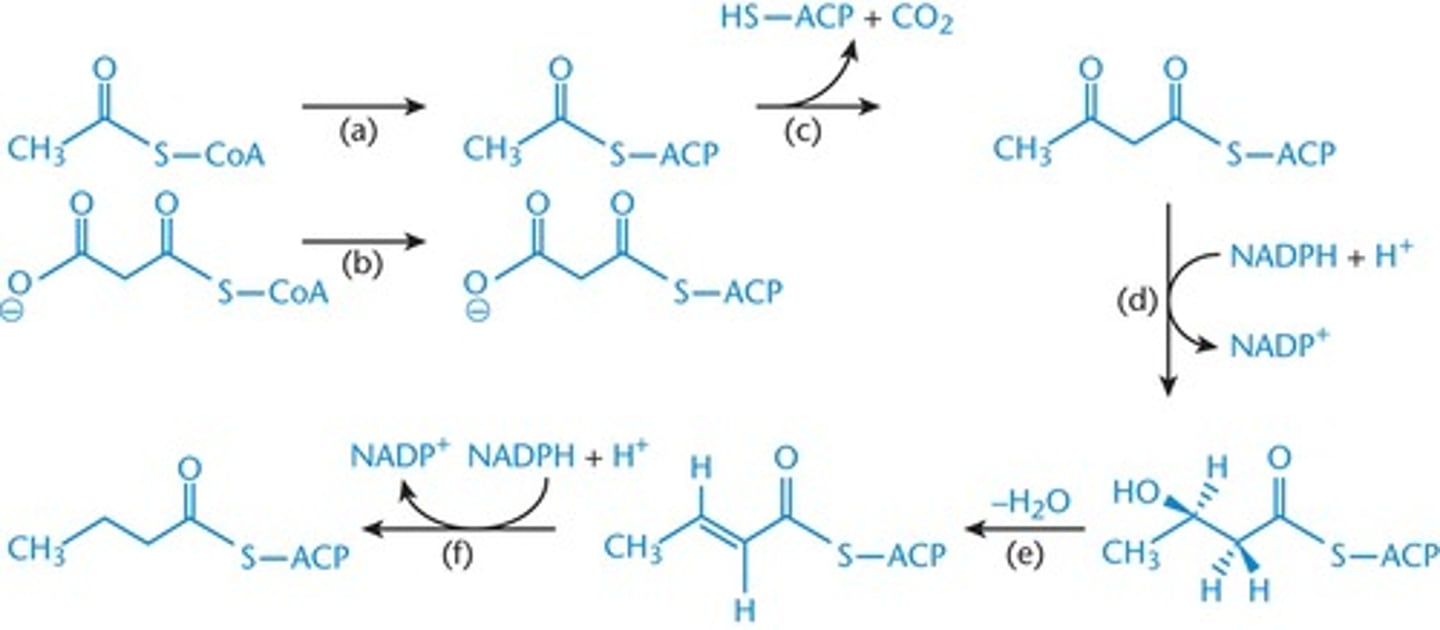
How many acetyl CoA groups are needed to produce palmitate (16:0)
eight
Other fatty acid catabolisms
-peroxisomal beta-oxidation
-alpha-oxidation of branched-chain fatty acids
- ω oxidation in the ER, producing dicarboyxlic acids
Triacylglycerol synthesis
-triacylglycerols = storage form of fatty acids
-formed by attaching three fatty acids (as fatty acyl-CoA) to glycerol
-occurs primarily in the liver (then packaged and sent to adipose in VLDL) and somewhat in adipose tissue
beta-oxidatoin
-primary form of fatty acid catabolism
-occurs in the mitochondria (also sometimes in peroxisome)
-stimulated by glucagon
-inhibited by insulin
-opposite of fatty acid synthesis, by understaning 1 process you can answer questions about both
steps of beta oxidation process?
activation
faty acid entry into mitochondria
beta oxidationin mitochondria
Carnitine acyltransferase I
rate limiting enzyme of fatty acid oxidation
Fatty-acyl-CoA synthetase
-activates fatty acids by attachment to CoA
-product is referred to as fatty acyl-CoA or acyl CoA (ie palmitoyl-CoA has 16 carbon acyl group)
Fatty acid entry into mitochondria
-short-chain fatty acids (2-4 carbons) and medium chain fatty acids (6-12) carbons diffuse freely into mitochondria
-long-chain fatty acids require transport via a carnitine shuttle
Note: very long chain fatty acids (20+ carbons) are oxidized elsewhere
Beta oxidation overview
oxidizes and releases molecules of acetyl-CoA (reverse of fatty acid synthesis.
has 4 steps. each 4 step cycle releases one acetyl CoA and reduces NAD+ and FAD (producing NADH and FADH2 which are oxidized in the e- transport chain)
fate of acetyl CoA in different places?
in muscle and adipose = enters citric acid cycle
in liver = stimulates gluconeogeneiss by activating pyruvate carboxylase (remember, it can't be converted back to glucose)
In fasting state = liver produces more acetyl CoA from beta oxidation than is used in TCA cycle so it is used to make ketone bodies (basically 2 acetyl CoA molecules linked together)
Propionyl-CoA carboxylase
-converts propionyl-CoA to methylmalonyl-CoA
-requires biotin (vitamin B7)
Four steps of beta-oxidation
1.oxidation of fatty acid to form a double bond (creates FADH2)
2. hydration of the double bond to form a hydroxyl group (req. H2O)
3. oxidation of the hydroxyl group to form a carbonyl; called a beta-ketoacid (generates NADH)
4. splitting of the beta-ketoacid into a shorter acyl-CoA and acetyl-CoA (req. CoA-SH)
-process continues until chain has been shortened to two carbons (for even chained fatty acids)
Odd-numbered chain oxidation
-final cycle = yields one acetyl-CoA and one propionyl-CoA from the final 5-carbon fragment
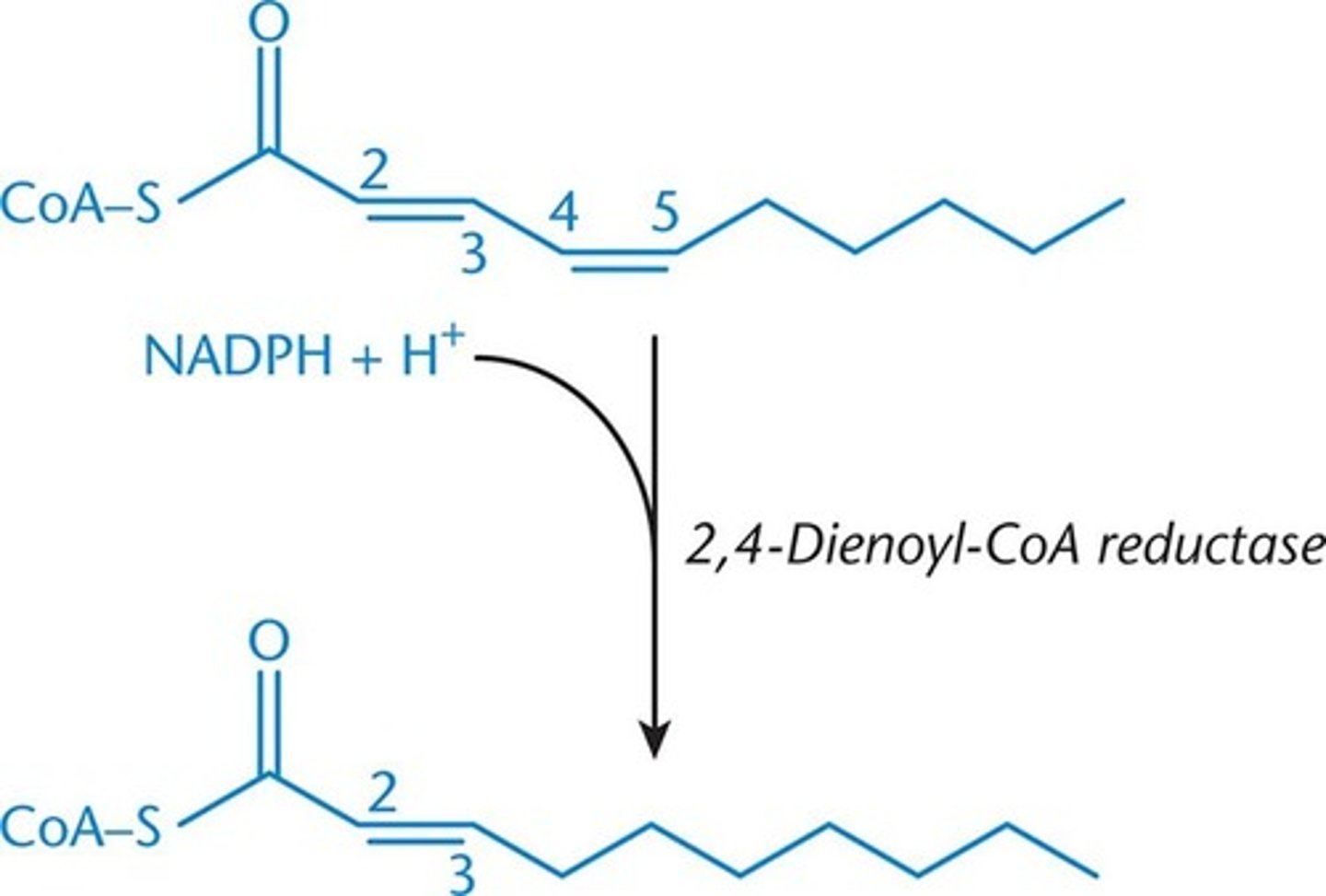
2,4-dienoyl-CoA reductase
-used in polyunsaturated fatty acids
-convert two conjugated double bonds to just one double bond at the 3,4 position, where it will undergo same rearrangement as monounsaturated
Methylmalonyl-CoA mutase
-converts methylmalonyl-CoA to succinyl-CoA
-requires cobalamin (vitamin B12)
-succinyl-CoA can go through TCA or be converted to malate to enter gluconeogenic pathway in cytosol
break down of saturated vs unsaturated fatty acids
previous processes = for saturated fatty acids
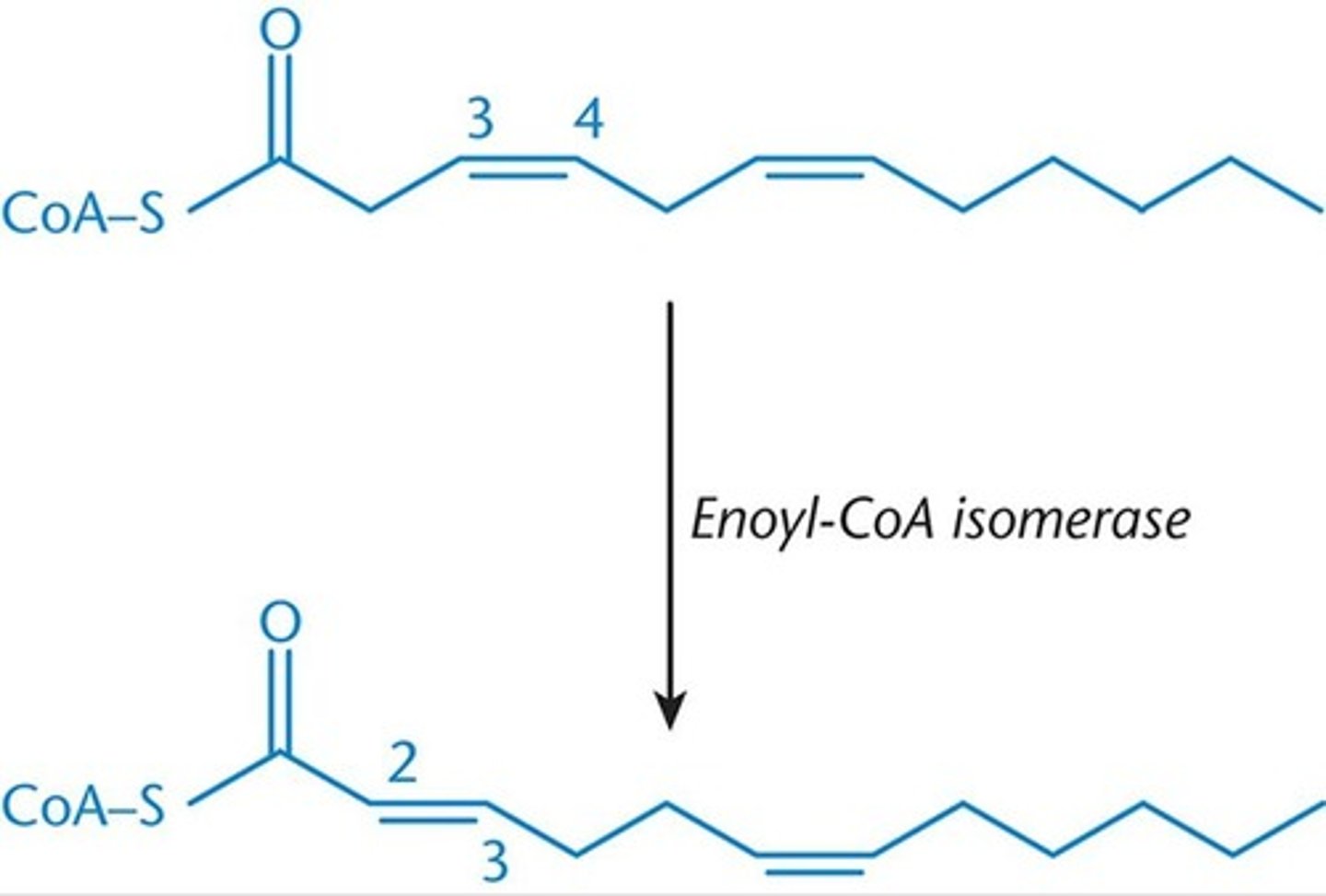
unsaturated fatty acids = need 2 additional enzymes bc double bonds disturb stereochemistry needed for oxidative enzymes to act on the fatty acid.
to function, these 2 enzymes can have at most 1 double bond in their active site and the bond MUST be located between carbons 2 and 3
2 enzymes = enoyl-CoA isomerase and 2,4-dienoyl CoA reductase
Ketone bodies
-formed in the fasting state, when liver converts excess acetyl-CoA into ketone bodies
-the ketone bodies are acetoacetate and 3-hydroxybutyrate
-can be used for energy in various tissues
Enoyl-CoA isomerase
-used in monounsaturated fatty acids
-rearranges cis double bonds at the 3,4 position to trans double bonds at the 2,3 positon once enough acetyl-CoA has been liberated to isolate the double bond w/in the first 3 carbons
HMG-CoA synthase
forms HMG-CoA from acetyl-CoA
HMG-CoA lyase
breaks down HMG-CoA into acetoacetate, which can subsequently be reduced to 3-hydroxybutyrate
Ketogenesis
-occurs in the mitochondria of liver cells when excess acetyl-CoA accumulates in the fasting state
-requires the enzyme HMG-CoA synthase and HMG-CoA lyase
-acetone = minor side product, not used as energy in tissues
Brain can derive...
up to 2/3 of its energy from ketone bodies during prolonged starvation
Ketolysis
-regenerates acetyl-Coa for use as an energy source in peripheral tissues
-requires the enzyme succinyl-CoA acetoacetyl-CoA transferase (thiophorase), which is only found in tissues outside the liver
-3-hydroxybutyrate is oxidized to acetoacetate
what does succinyl-CoA acetoacetyl-CoA transferase (thiophorase) do?
activates the acetoacetate = converts acetoacetate to acetoacetyl-CoA (which can be broken down into 2 aceytl-CoA)
does protein digestion happen frequently for metabolism?
NO! metabolism is directed toward conserving tissues, esp heart and brain
digestion of proteins compromises muscle (potentially the heart) so its unlikely to occur under normal circomstances.
also proteins are v. important in other functions
Urea cycle
-occurs in the liver
-primary way of removing excess nitrogen from the body
digstion of prteins begins where?
in stomach w/ pepsin.
continues w/ pancreatic proteases: trypsin, chymotrypsin, and carboxypeptidases A and B (all are secreted as zymogens)
Protein digestion is completed where? enzymes (2)? products
the small intestine by the brush-border enzymes dipeptidase and aminopeptidase
products = amino acids, di/tripeptides
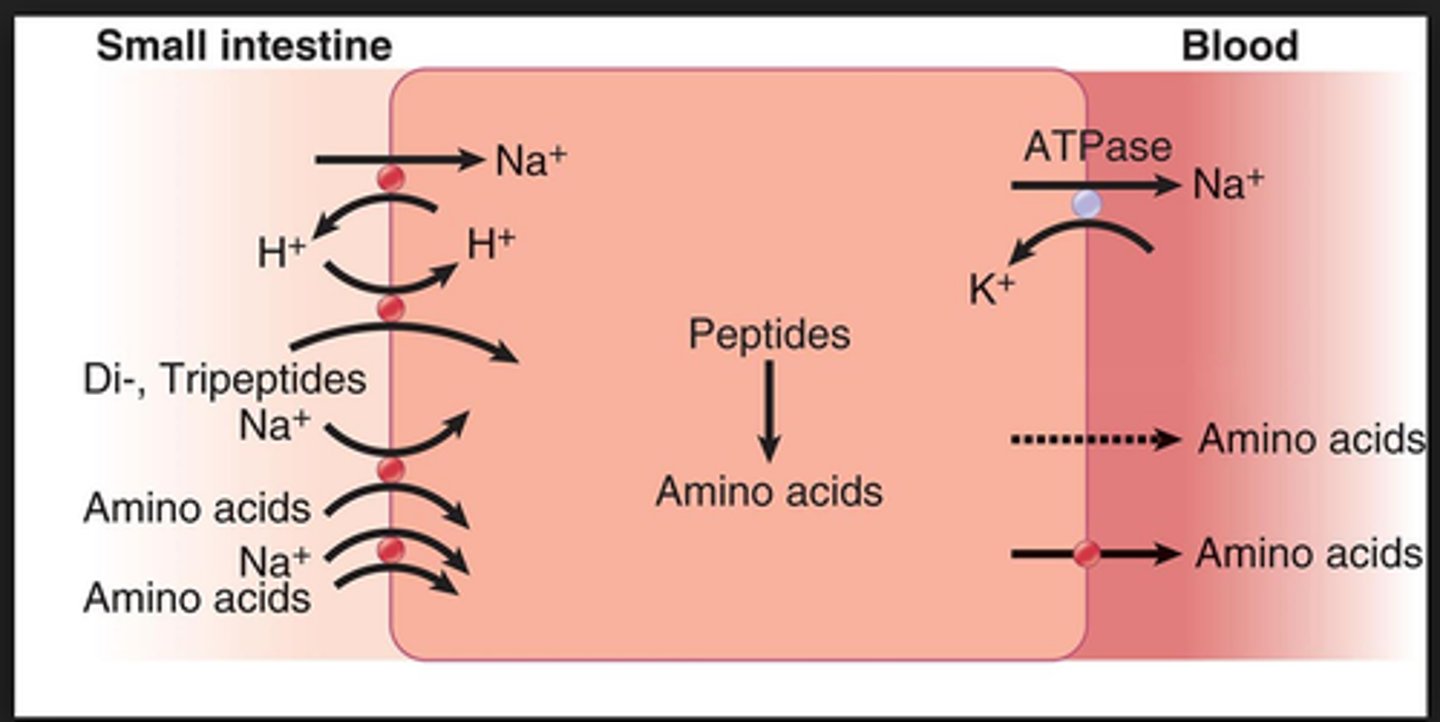
absorption of amino acids/small peptides = ?
thru luminal membrane via secondary active transport linked to sodium
at the basal membrane = simple and facilitated diffusion transport AAs into bloodstream
body protein is catabolized mainly where?
muscle and liver
Carbon skeletons are used for...
energy, either through gluconeogenesis or ketone body formation
Transamination
loss of an amino group from the amino acid. remaining carbon skeleton can be used for energy
loss of an amino group from the amino acid can also happen via deamination
Classification of AAs in catabolism
classified by their ability to turn into specifc metabolic intermediates
-glucogenic AAs (all but leucine and lysine) = can be converted into glucose via gluconeogenesis
-ketogenic AAs (L,K,I,F,T,W,Y) = can be converted to acetyl-CoA and ketone bodies
amino group removed by trans/de-amination = potential toxen, how? How is it taken care of?
could turn into ammonia?
excreted via urea cycle
Catabolism of cellular proteins occurs only under...
conditions of starvation
fate of side chains?
basic AA side chains feed into urea cycle
other side chains act like carbon skeletons and produce energy via gluconeogenesis or ketone production