Embryo: The Eye
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
25 Terms
Describe the formation of the eyes, the eyelids and the lacrimal glands.
Explain the embryological basis for the most common congenital anomalies of the eye.
Langman's Medical Embryology
Chapter 20, Eye
OPTIC CUP AND LENS VESICLE
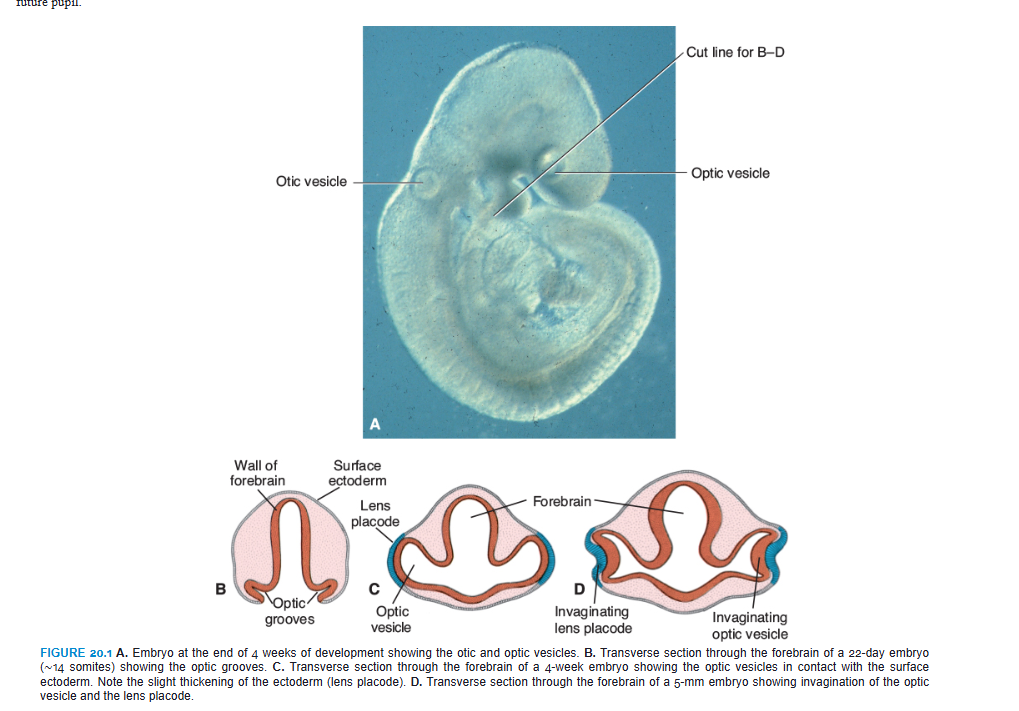
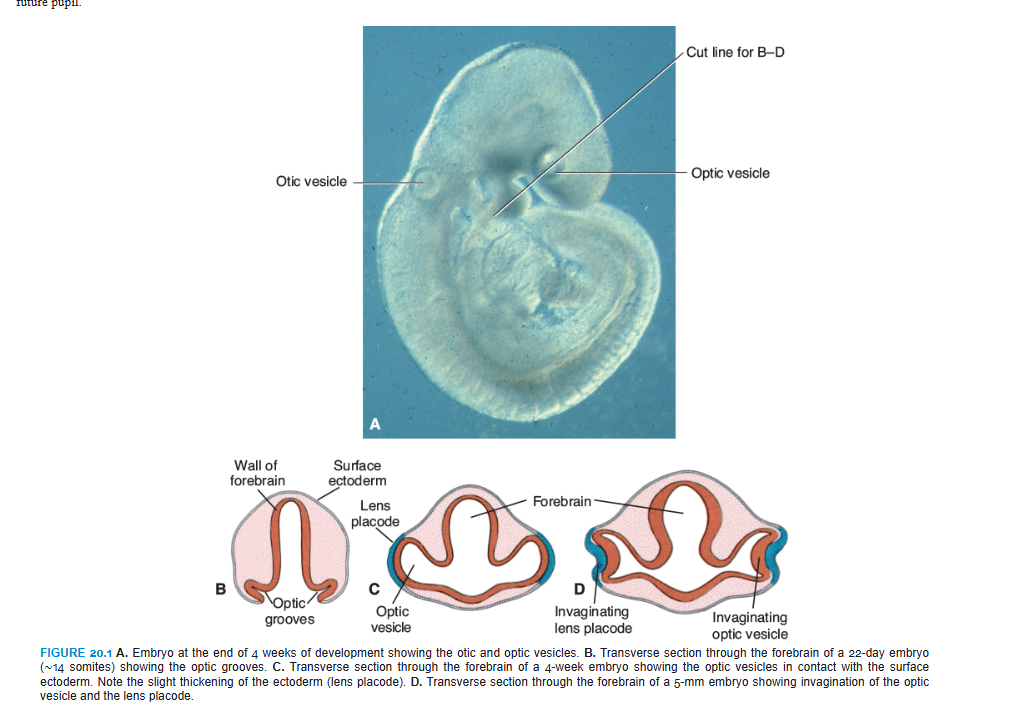
1. developing eye appears in the 22nd day as a pair of shallow grooves on the sides of the forebrain
2. neural tube closes
3. the shallow grooves form the outpocketings of the forebrain (the optic vesicles).
4. optic vesicles come in contact with the surface ECTOderm and the optic vesicles induce changes in the ectoderm necessary for lens formation.
5. optic vesicles begin to invaginate and form the double walled optic cup.
6. inner and outer layers of the cup are separated by a lumen (lumen= intraretinal space)
7. lumen disappears, and the two layers oppose each other.
8. invagination not only restricted to the central portion of the cup, but also involves part of the inferior surface of the cup.
the inferior surface of the cup forms the choroid fissure.
10. formation of the fissure allows the hyoid artery to reach the inner chamber of the eye.
11. during the seventh week, the lips of the choroid fissure fuse, and the mouth of the optic cup becomes a round opening, the future pupil.
during events 1-11, the cells of the surface ECTODERM, initially in contact with the optic vesicle, begin to elongate and form the lens placode.
this placode subsequently invaginates and develops into the lens vesicle.
during the fifth week, the lens vesicle loses contact with the surface ectoderm and lies in the mouth of the optic cup.
RETINA, IRIS, AND CILIARY BODY
pigmented layer of the retina
The outer layer of the optic cup, which is characterized by small pigment granules, is known as the pigmented layer of the retina (Fig. 20.2D,E; see also Fig. 20.6).
development of the inner (neural layer) of the optic cup
Development of the inner (neural) layer of the optic cup is more complicated.
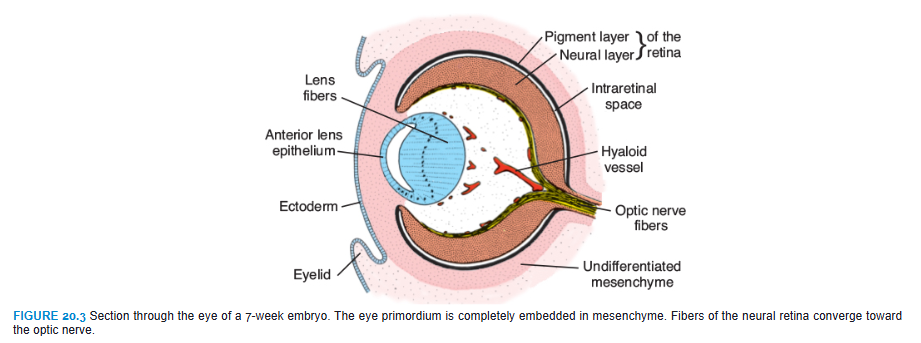
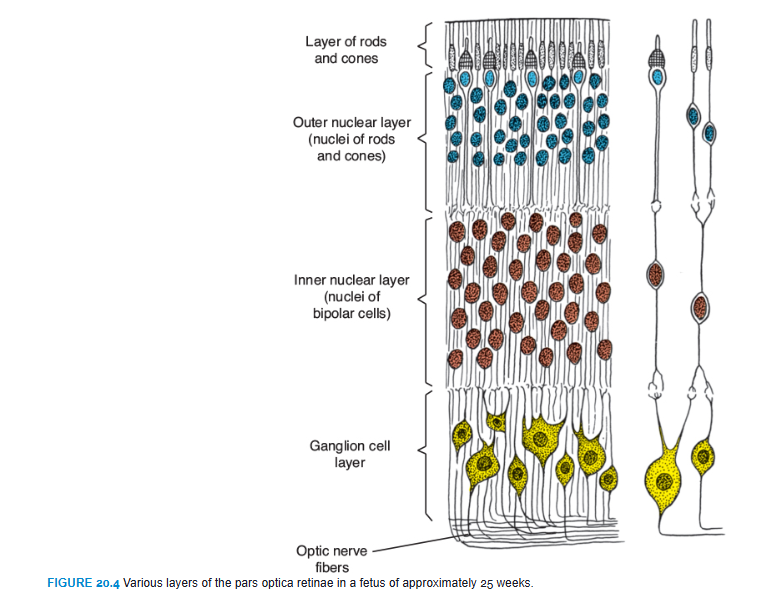
The posterior four-fifths, the pars optica retinae, contains cells bordering the intraretinal space (Fig. 20.3) that differentiate into the photoreceptive rods and cones (Fig. 20.4). Rods are more numerous (120 million) and more sensitive than cones (6 to 7 million) but do not detect color like the cones. Adjacent to this photoreceptive layer is the mantle layer, which, as in the brain, gives rise to neurons and supporting cells, including the outer nuclear layer, inner nuclear layer, and ganglion cell layer (Fig. 20.4). On the surface is a fibrous layer that contains axons of nerve cells of the deeper layers. Nerve fibers in this zone converge toward the optic stalk, which develops into the optic nerve (Fig. 20.3). Hence, light impulses pass through most layers of the retina before they reach the rods and cones.
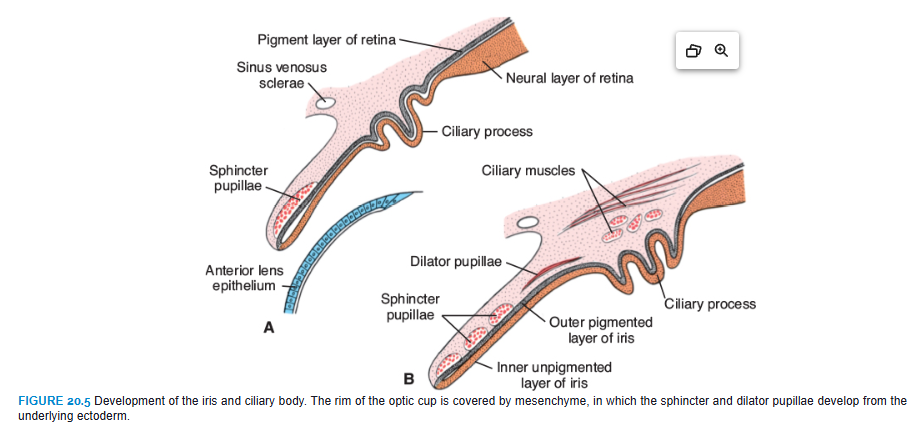
The anterior fifth of the inner layer, the pars ceca retinae, remains one cell layer thick. It later divides into the pars iridica retinae, which forms the inner layer of the iris, and the pars ciliaris retinae, which participates in formation of the ciliary body (Figs. 20.5 and 20.6).
Meanwhile, the region between the optic cup and the overlying surface epithelium is filled with loose mesenchyme (Figs. 20.2C and 20.6). The sphincter and dilator pupillae muscles form in this tissue (Fig. 20.5). These muscles develop from the underlying ectoderm of the optic cup. In the adult, the iris is formed by the pigment-containing external layer, the unpigmented internal layer of the optic cup, and a layer of richly vascularized connective tissue that contains the pupillary muscles (Fig. 20.5).
The pars ciliaris retinae is easily recognized by its marked folding (Figs. 20.5B and 20.6). Externally, it is covered by a layer of mesenchyme that forms the ciliary muscle; on the inside, it is connected to the lens by a network of elastic fibers, the suspensory ligament or zonula (Fig. 20.6). Contraction of the ciliary muscle changes tension in the ligament and controls curvature of the lens.
LENS
Shortly after formation of the lens vesicle (Fig. 20.2C), cells of the posterior wall begin to elongate anteriorly and form long fibers that gradually fill the lumen of the vesicle (Fig. 20.3). By the end of the seventh week, these primary lens fibers reach the anterior wall of the lens vesicle. Growth of the lens is not finished at this stage, however, because new (secondary) lens fibers are continuously added to the central core.
CHOROID, SCLERA, AND CORNEA
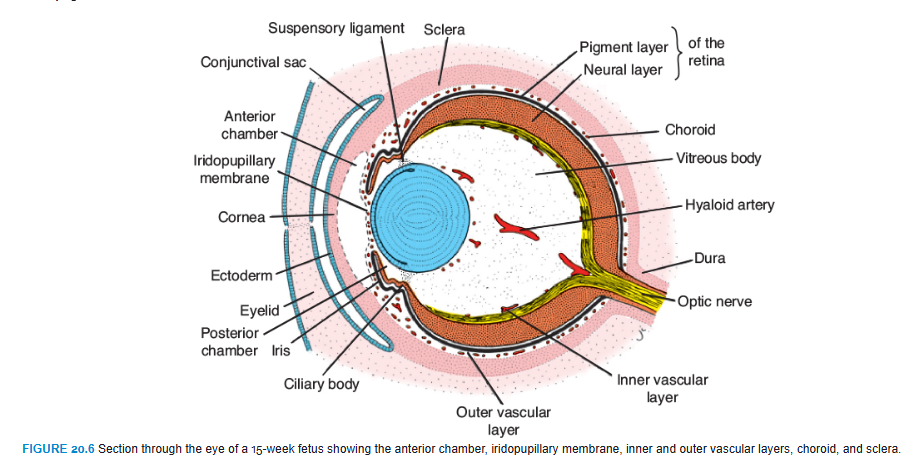
At the end of the fifth week, the eye primordium is completely surrounded by loose mesenchyme (Fig. 20.3). This tissue soon differentiates into an inner layer comparable with the pia mater of the brain and an outer layer comparable with the dura mater. The inner layer later forms a highly vascularized pigmented layer known as the choroid; the outer layer develops into the sclera and is continuous with the dura mater around the optic nerve (Fig. 20.6).
Differentiation of mesenchymal layers overlying the anterior aspect of the eye is different. The anterior chamber forms through vacuolization and splits the mesenchyme into an inner layer in front of the lens and iris, the iridopupillary membrane, and an outer layer continuous with the sclera, the substantia propria of the cornea (Fig. 20.6). The anterior chamber itself is lined by flattened mesenchymal cells. Hence, the cornea is formed by (1) an epithelial layer derived from the surface ectoderm; (2) the substantia propria or stroma, which is continuous with the sclera; and (3) an epithelial layer, which borders the anterior chamber. The iridopupillary membrane in front of the lens disappears completely. The posterior chamber is the space between the iris anteriorly and the lens and ciliary body posteriorly. The anterior and posterior chambers communicate with each other through the pupil and are filled with fluid called the aqueous humor produced by the ciliary process of the ciliary body. The clear aqueous humor circulates from the posterior chamber into the anterior chamber providing nutrients for the avascular cornea and lens. From the anterior chamber, the fluid passes through the scleral venous sinus (canal of Schlemm) at the iridocorneal angle where it is resorbed into the bloodstream. Blockage of the flow of fluid at the canal of Schlemm is one cause of glaucoma
VITREOUS BODY
Mesenchyme not only surrounds the eye primordium from the outside but also invades the inside of the optic cup by way of the choroid fissure. Here, it forms the hyaloid vessels, which during intrauterine life supply the lens and form the vascular layer on the inner surface of the retina (Fig. 20.6). In addition, it forms a delicate network of fibers between the lens and retina. The interstitial spaces of this network later fill with a transparent gelatinous substance, forming the vitreous body (Fig. 20.6). The hyaloid vessels in this region are obliterated and disappear during fetal life, leaving behind the hyaloid canal.
OPTIC NERVE
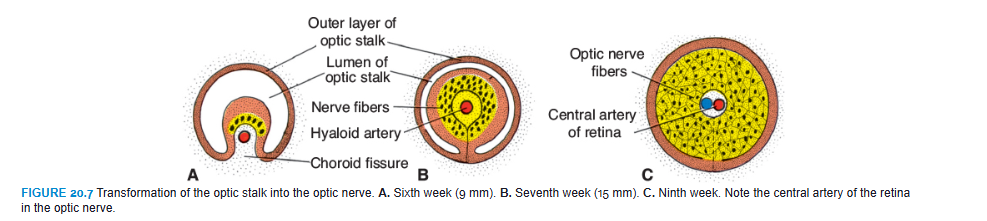
The optic cup is connected to the brain by the optic stalk, which has a groove, the choroid fissure, on its ventral surface (Figs. 20.2 and 20.3). In this groove are the hyaloid vessels. The nerve fibers of the retina returning to the brain lie among cells of the inner wall of the stalk (Fig. 20.7). During the seventh week, the choroid fissure closes, and a narrow tunnel forms inside the optic stalk (Fig. 20.7B). As a result of the continuously increasing number of nerve fibers, the inner wall of the stalk grows, and the inside and outside walls of the stalk fuse (Fig. 20.7C). Cells of the inner layer provide a network of neuroglia that supports the optic nerve fibers.
The optic stalk is thus transformed into the optic nerve. Its center contains a portion of the hyaloid artery, later called the central artery of the retina. On the outside, a continuation of the choroid and sclera, the pia arachnoid and dura layer of the nerve, respectively, surround the optic nerve.
MOLECULAR REGULATION OF EYE DEVELOPMENT
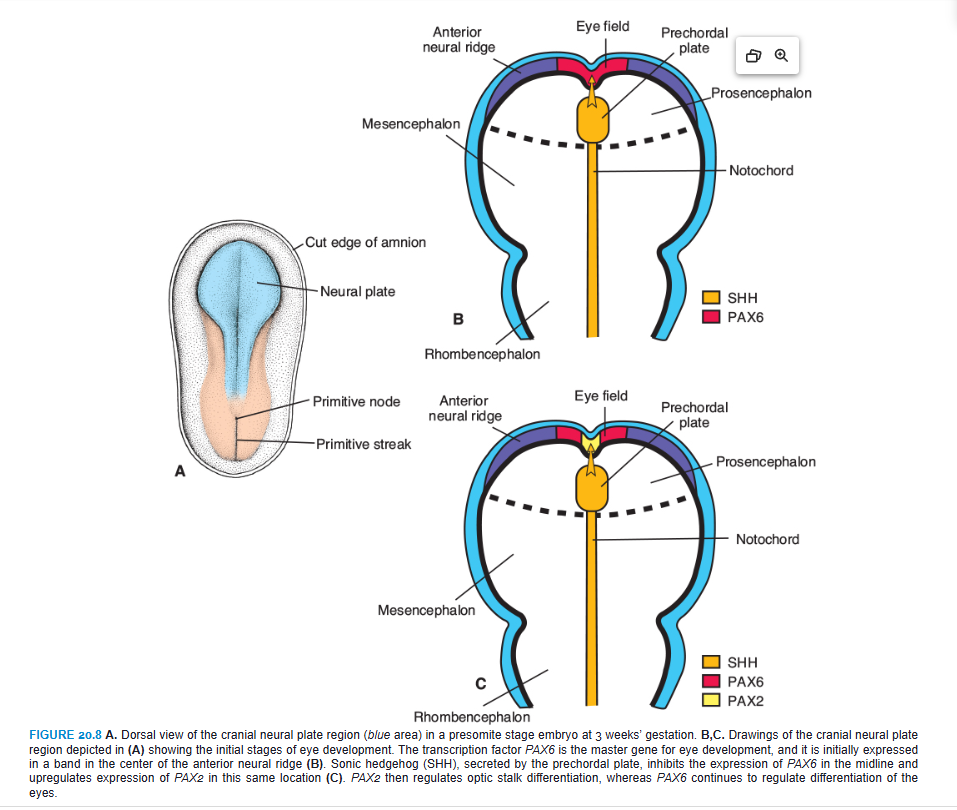
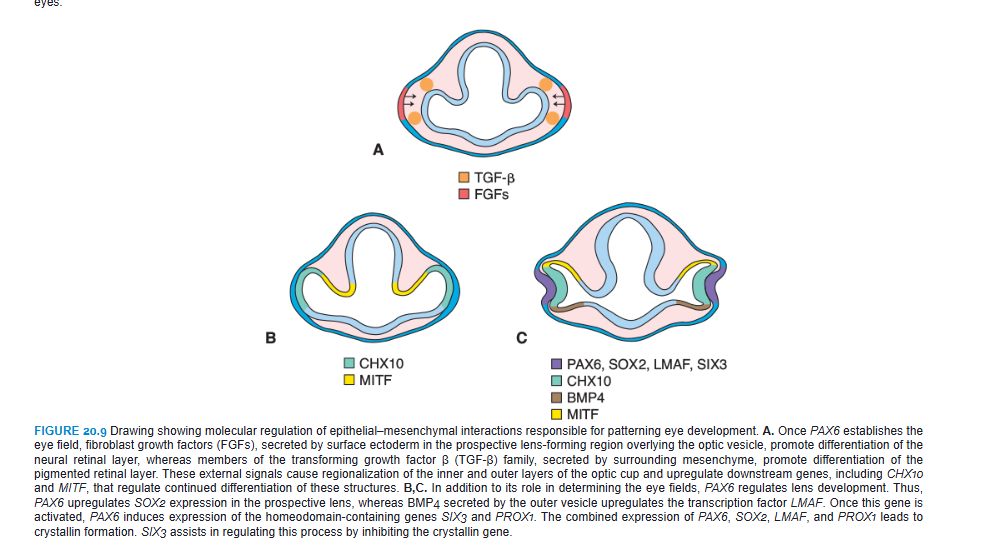
PAX6 is the key regulatory gene for eye development. It is a member of the PAX (paired box) family of transcription factors and contains two DNA-binding motifs that include a paired domain and a paired-type homeodomain. Initially, this transcription factor is expressed in a band in the anterior neural ridge of the neural plate before neurulation begins (Fig. 20.8A,B; see also Fig. 18.32). At this stage, there is a single eye field that later separates into two optic primordia (Fig. 20.8B). The signal for separation of this field is SONIC HEDGEHOG (SHH) expressed in the prechordal plate. SHH expression upregulates PAX2 in the center of the eye field and downregulates PAX6 (Fig. 20.8C). Later, this pattern is maintained so that PAX2 is expressed in the optic stalks and PAX6 is expressed in the optic cup and overlying surface ectoderm that forms the lens. As development proceeds, it appears that PAX6 is not essential for optic cup formation. Instead, this process is regulated by interactive signals between the optic vesicle and surrounding mesenchyme and the overlying surface ectoderm in the lens-forming region (Fig. 20.9). Thus, fibroblast growth factors (FGFs) from the surface ectoderm promote differentiation of the neural (inner layer) retina, whereas transforming growth factor β (TGF-β), secreted by surrounding mesenchyme, directs formation of the pigmented (outer) retinal layer. Downstream from these gene products, the transcription factors MITF and CHX10 are expressed and direct differentiation of the pigmented and neural layer, respectively (Fig. 20.9). Thus, the lens ectoderm is essential for proper formation of the optic cup, such that without a lens placode, no cup invagination occurs.
Differentiation of the lens depends on PAX6, although the gene is not responsible for inductive activity by the optic vesicle. Instead, PAX6 acts in the surface ectoderm to regulate lens development (Fig. 20.9C). This expression upregulates the transcription factor SOX2 and also maintains PAX6 expression in the prospective lens ectoderm. In turn, the optic vesicle secretes BMP4, which also upregulates and maintains SOX2 expression as well as expression of LMAF, another transcription factor (Fig. 20.9C). Next, the expression of two homeobox genes, SIX3 and PROX1, is regulated by PAX6. The combined expression of PAX6, SOX2, and LMAF initiates expression of genes responsible for lens crystallin formation, including PROX1. SIX3 also acts as a regulator of crystallin production by inhibiting the crystallin gene. Finally, PAX6, acting through FOX3, regulates cell proliferation in the lens.
Eye Abnormalities
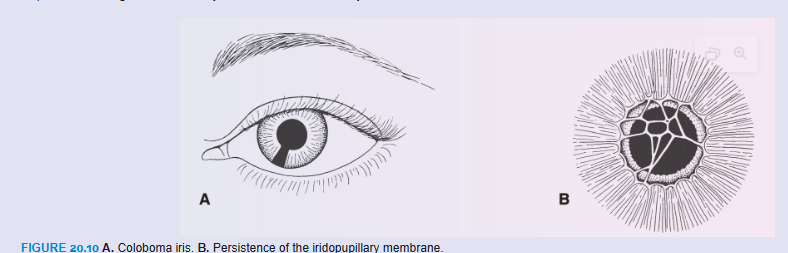
Coloboma may occur if the choroid fissure fails to close. Normally, this fissure closes during the seventh week of development (Fig. 20.7). When it does not, a cleft persists. Although such a cleft is usually in the iris only—coloboma iridis (Fig. 20.10A)—it may extend into the ciliary body, the retina, the choroid, and the optic nerve. Coloboma is a common eye abnormality frequently associated with other eye defects. Colobomas (clefts) of the eyelids may also occur. Mutations in the PAX2 gene have been linked with optic nerve colobomas and may play a role in the other types as well. Renal defects also occur with mutations in PAX2 as part of the renal coloboma syndrome (see Chapter 16, p. 264).
The iridopupillary membrane (Fig. 20.10B) may persist instead of being resorbed during formation of the anterior chamber.
Congenital cataracts cause the lens to become opaque during intrauterine life. Although this anomaly is usually genetically determined, many children born to mothers who had rubella (German measles) between the fourth and seventh weeks of pregnancy had cataracts. If the mother is infected after the seventh week of pregnancy, the lens escapes damage, but the child may have hearing loss as a result of cochlear abnormalities. Because of the MMR (measles, mumps, and rubella) vaccine, congenital rubella syndrome has been nearly eradicated in the United States.
The hyaloid artery may persist to form a cord or cyst. Normally, the distal portion of this vessel degenerates, leaving the proximal part to form the central artery of the retina.
In microphthalmia, the eye is too small; the eyeball may be only two-thirds of its normal volume. Usually associated with other ocular abnormalities, microphthalmia can result from intrauterine infections, such as cytomegalovirus and toxoplasmosis.
Anophthalmia is absence of the eye. In some cases, histologic analysis reveals some ocular tissue. The defect is usually accompanied by severe cranial abnormalities.
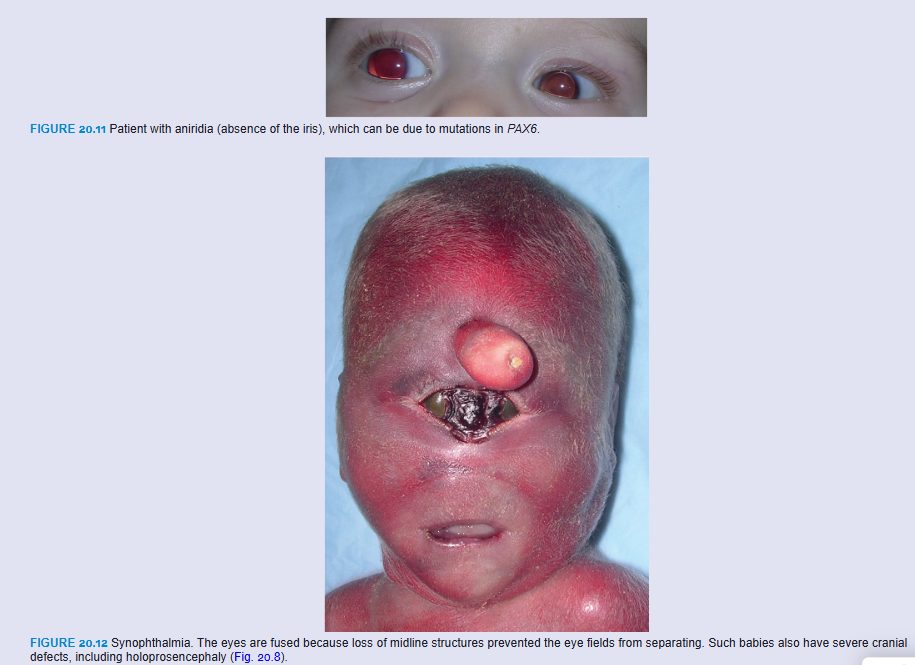
Congenital aphakia (absence of the lens) and aniridia (absence of the iris; Fig. 20.11) are rare anomalies that result from disturbances in induction and development of tissues responsible for formation of these structures. Mutations in PAX6 result in aniridia and may also contribute to anophthalmia and microphthalmia.
Cyclopia (single eye) and synophthalmia (fusion of the eyes) comprise a spectrum of defects in which the eyes are partially or completely fused (Fig. 20.12). The defects are caused by a loss of midline tissue that may occur as early as days 19 to 21 of gestation or at later stages when facial development is initiated. This loss results in underdevelopment of the forebrain and frontonasal prominence. These defects are invariably associated with a brain defect called holoprosencephaly, in which the cerebral hemispheres are partially or completely merged into a single telencephalic vesicle. Factors associated with holoprosencephaly include maternal alcohol use, maternal diabetes, mutations in SHH, and abnormalities in cholesterol metabolism that may disrupt SHH signaling (see Chapter 18).
SUMMARY
The eyes begin to develop as a pair of outpocketings that will become the optic vesicles on each side of the forebrain at the end of the fourth week of development (Fig. 20.1). The optic vesicles contact the surface ectoderm and induce lens formation. When the optic vesicle begins to invaginate to form the pigment and neural layers of the retina, the lens placode invaginates to form the lens vesicle. Through a groove at the inferior aspect of the optic vesicle, the choroid fissure, the hyaloid artery (later the central artery of the retina) enters the eye (Fig. 20.3). Nerve fibers of the eye also occupy this groove to reach the optic areas of the brain. The cornea is formed by (1) a layer of surface ectoderm; (2) the stroma, which is continuous with the sclera; and (3) an epithelial layer bordering the anterior chamber (Fig. 20.6).
PAX6, the master gene for eye development, is expressed in the single eye field at the neural plate stage. The eye field is separated into two optic primordia by SHH, which upregulates PAX2 expression in the optic stalks while downregulating PAX6, restricting this gene’s expression to the optic cup and lens. Epithelial–mesenchymal interactions between prospective lens ectoderm, optic vesicle, and surrounding mesenchyme then regulate lens and optic cup differentiation (Figs. 20.8 and 20.9).
Problems to Solve
A newborn has unilateral aphakia (absent lens). What is the embryologic origin of this defect?
In taking a history of a young woman in her 10th week of gestation, you become concerned that she may have contracted rubella sometime during the fourth to eighth weeks of her pregnancy. What types of defects might be produced in her offspring?
Physical examination of a newborn reveals clefts in the lower portion of the iris bilaterally. What is the embryologic basis for this defect? What other structures might be involved?
question from class
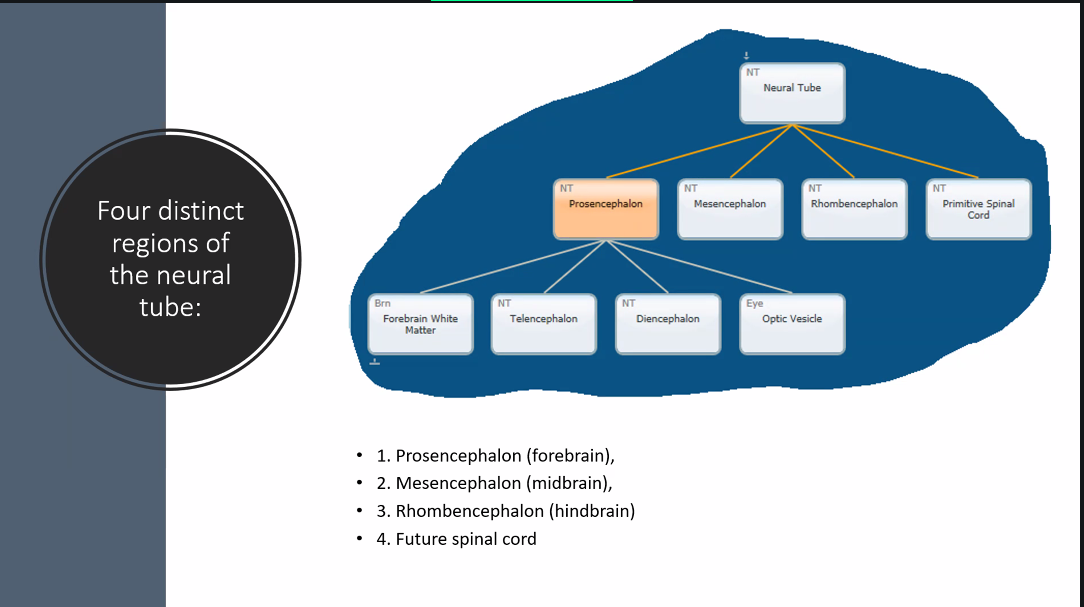
what does the neural tube become?
the spinal cord and brain.
be familiar with the four different divisions of the brain and their development
A)
prosencephalon
gives rise to the midbrain.
D)
choroid: membrane/skin
fissure: crack
choroid fissure: the split (groove) where the choroid-like membrane attaches

C)

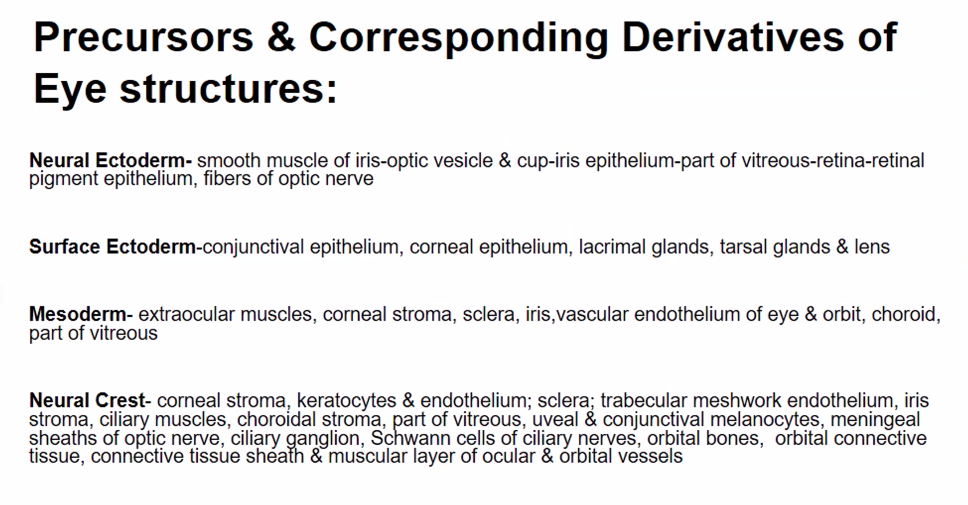
A)