AP Bio - Chapter 2 (Chemistry)
5.0(2)
Card Sorting
1/49
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
50 Terms
1
New cards
Atoms
the smallest unit of matter that contains all of the chemical properties of a certain element. For example, a carbon atom exhibits the properties of carbon.
made of two regions: nucleus (with protons and neutrons) and outermost region (with electrons)
made of two regions: nucleus (with protons and neutrons) and outermost region (with electrons)
2
New cards
Isotopes
one or more types of an element that have different numbers of neutrons
some elements have naturally occuring ________
radioactive ones emit neutrons, protons, and electrons to attain a more stable atomic configuration (lower level of potential energy)
some elements have naturally occuring ________
radioactive ones emit neutrons, protons, and electrons to attain a more stable atomic configuration (lower level of potential energy)
3
New cards
Carbon dating
C^14 is an isomer of carbon that is radioactive (naturally occurs)
used to figure out the date of living things’ life
\
used to figure out the date of living things’ life
\
4
New cards
Periodic Table
a chart of the elements that tells the atomic number and atomic mass of each element; gives information about the properties of each element
made by Dmitri Mendeleev
organized according to atomic # and arranged in rows and columns based on shared chemical and physical properties
made by Dmitri Mendeleev
organized according to atomic # and arranged in rows and columns based on shared chemical and physical properties
5
New cards
Chemical reactivity
the ability to combine and to chemically bond with each other
6
New cards
Molecules
two or more atoms that are chemically bonded together
7
New cards
Bohr Model
shows the atom as a central nucleus containing protons and neutrons, with the electrons in circular orbitals at specific distances from the nucleus
orbits form electron shells/energy levels
fill the orbitals closest to the nucleus first then move outward
multiple orbitals of equal energy= filled with one electron in each energy level before a second electron is added
electrons in outermost shell determine the stability of the atom and its tendency to form chemical bonds
\*see pg. 59 diagram in textbook
orbits form electron shells/energy levels
fill the orbitals closest to the nucleus first then move outward
multiple orbitals of equal energy= filled with one electron in each energy level before a second electron is added
electrons in outermost shell determine the stability of the atom and its tendency to form chemical bonds
\*see pg. 59 diagram in textbook
8
New cards
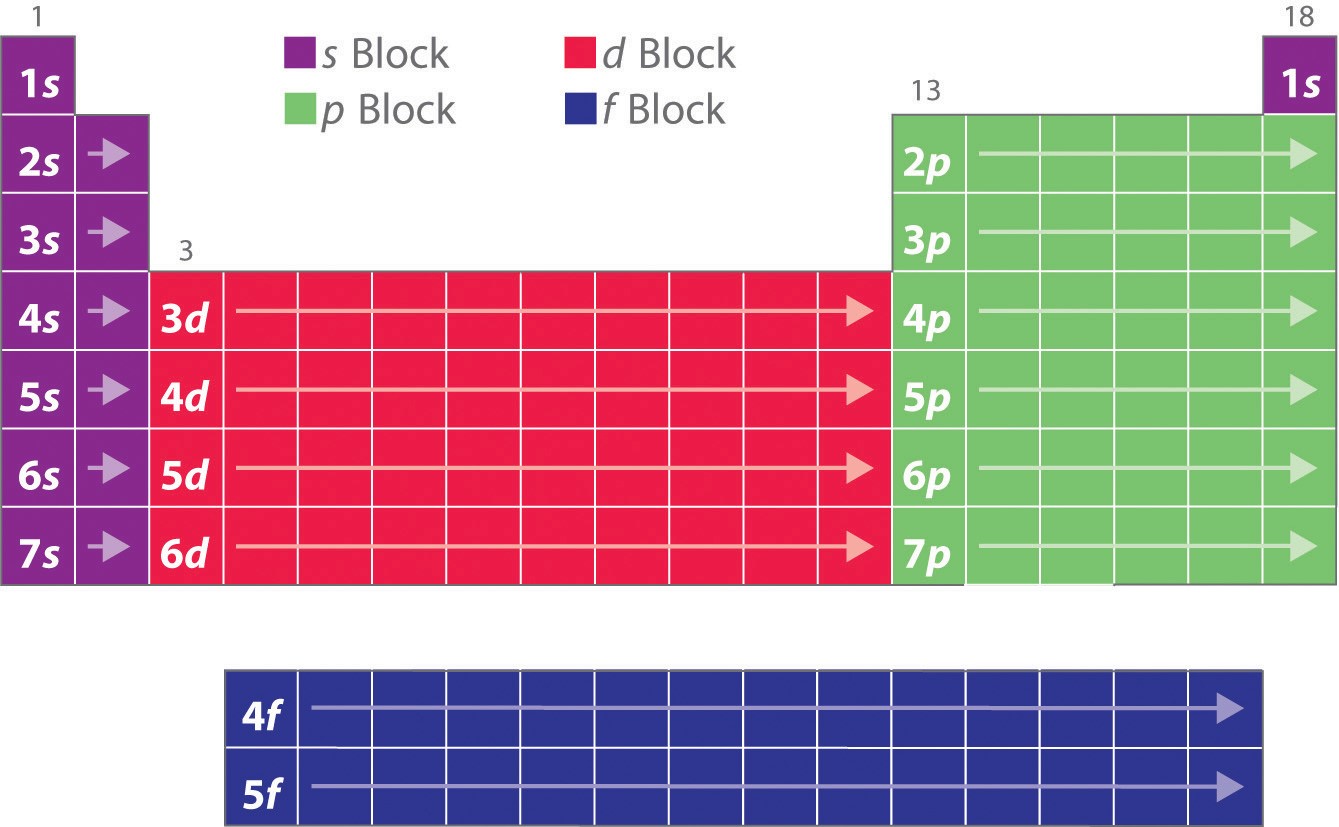
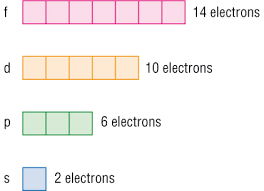
Orbitals
\
inside shells are subshells (s, p, d, f) ex: Li is 1s²2s¹
be able to look at a drawing and tell if it is correct.
the area that contains the electrons outside of the nucleus
inside shells are subshells (s, p, d, f) ex: Li is 1s²2s¹
be able to look at a drawing and tell if it is correct.
the area that contains the electrons outside of the nucleus
9
New cards
Electron configuration
\
the arrangement of electrons in an atom’s electron shell. Example: 1s^2,
2s^2, 2p^6 is Neon
the arrangement of electrons in an atom’s electron shell. Example: 1s^2,
2s^2, 2p^6 is Neon
10
New cards
Reactants
molecule that is on the left side of a chemical equation
11
New cards
Reversible chemical reactions
chemical reaction where the product may turn back into the reactants if their concentration is great enough
back and forth continues until equilibrium is reached
shown by a double headed arrow
back and forth continues until equilibrium is reached
shown by a double headed arrow
12
New cards
Equilibrium of reactants and products
\
can only put numbers in front of elements, cannot change the subscripts
a written chemical reaction with the number of each type of atom equal on both sides of the equation (both on the product side and the reactant side)
can only put numbers in front of elements, cannot change the subscripts
a written chemical reaction with the number of each type of atom equal on both sides of the equation (both on the product side and the reactant side)
13
New cards
Ionic bonds
\
a chemical bond that happens between ions with differing charges (cations and anions)
when an atom gives away an electron or gains an electron
create a zero net charge
a chemical bond that happens between ions with differing charges (cations and anions)
when an atom gives away an electron or gains an electron
create a zero net charge
14
New cards
Electrolytes
\
ion needed for nerve communication, muscle contractions, and water balance in the body.
exs: sodium, potassium, and calcium
ion needed for nerve communication, muscle contractions, and water balance in the body.
exs: sodium, potassium, and calcium
15
New cards
Covalent bonds
\
type of strong bond formed when valence electrons are shared between two atoms of the same or different elements
more common than ionic bonding
ex: formation of water molecules
type of strong bond formed when valence electrons are shared between two atoms of the same or different elements
more common than ionic bonding
ex: formation of water molecules
16
New cards
Single and double covalent bonds
one electron shared, two electrons shared
the more covalent bonds between two atoms, the stronger their connection
the more covalent bonds between two atoms, the stronger their connection
17
New cards
Polar covalent bonds
type of covalent bond where their is an unequal sharing of electrons, resulting in the creation of a slightly positive and slightly negative charged areas of the molecule
due to differences in electronegativity
ex: water (hydrogen atoms have a partial positive charge, oxygen has a partial negative charge) polarity allows for the formation of hydrogen bonds between adjacent water molecules= unique properties
due to differences in electronegativity
ex: water (hydrogen atoms have a partial positive charge, oxygen has a partial negative charge) polarity allows for the formation of hydrogen bonds between adjacent water molecules= unique properties
18
New cards
hydrogen bonding
\
a weak type of bond that is between a slightly positively charged hydrogen atom and a slightly negatively charged atom in other molecules
ex: water, DNA
a weak type of bond that is between a slightly positively charged hydrogen atom and a slightly negatively charged atom in other molecules
ex: water, DNA
19
New cards
Water properties
polar
high heat capacity
high kinetic energy
high heat of vaporization
hydrogen bonds
cohesive
adhesive
capillary action
universal solvent
pH is 7
states of water (gas, liquid, solid)
high heat capacity
high kinetic energy
high heat of vaporization
hydrogen bonds
cohesive
adhesive
capillary action
universal solvent
pH is 7
states of water (gas, liquid, solid)
20
New cards
pH
paper used is called litmus paper
based on water
stands for power of hydrogen
scale ranging from 0 to 14 that is proportional to the amount of hydrogen ions in a solution
acidity (1-6) or alkaline (8-14)
based on water
stands for power of hydrogen
scale ranging from 0 to 14 that is proportional to the amount of hydrogen ions in a solution
acidity (1-6) or alkaline (8-14)
21
New cards
Buffers
\
substance that stops a change in pH by absorbing or letting go of hydrogen or hydroxide ions. Example: bicarbonate in blood
maintains the correct pH (homeostasis)
internal pH of the body is between 6.5 and 7.5
is a divided compound
ex: peptobismol or antacids combat excess stomach acid
substance that stops a change in pH by absorbing or letting go of hydrogen or hydroxide ions. Example: bicarbonate in blood
maintains the correct pH (homeostasis)
internal pH of the body is between 6.5 and 7.5
is a divided compound
ex: peptobismol or antacids combat excess stomach acid
22
New cards
Isomers
\
molecules that differ from one another even though they share the same chemical formula; the arrangement of their atoms are different
ex: glucose and fructose are both C6H12O6 but their arrangement is different
structural ones differ in the placement of their covalent bonds (butane and isobutane)
geometric ones differ in how their bonds are made to the surrounding atoms
molecules that differ from one another even though they share the same chemical formula; the arrangement of their atoms are different
ex: glucose and fructose are both C6H12O6 but their arrangement is different
structural ones differ in the placement of their covalent bonds (butane and isobutane)
geometric ones differ in how their bonds are made to the surrounding atoms
23
New cards
Enantiomers
\
molecules that share overall structure and bonding patterns but differ in how the atoms are arranged three-dimensionally (they mirror each other)
molecules that share overall structure and bonding patterns but differ in how the atoms are arranged three-dimensionally (they mirror each other)
24
New cards
\
proton
proton
positively charged particle that stays in the nucleus of an atom; has a mass of one amu and a charge of 1+
shown by atomic number
count will not change and will always match the atomic number unless the element is bombarded or decays
shown by atomic number
count will not change and will always match the atomic number unless the element is bombarded or decays
25
New cards
neutron
subtract atomic number (protons) from atomic mass
uncharged particle that stays in the nucleus of an atom; has a mass of one amu
uncharged particle that stays in the nucleus of an atom; has a mass of one amu
26
New cards
electrons
shown by atomic number (same as protons)
negatively charged subatomic particle that remains outside of the nucleus, orbiting around the nucleus; has no mass and a negative charge of -1
negatively charged subatomic particle that remains outside of the nucleus, orbiting around the nucleus; has no mass and a negative charge of -1
27
New cards
n
energy level sublevels
ex: 1n is the first energy level (closest to the nucleus)
part of the Bohr model
ex: 1n is the first energy level (closest to the nucleus)
part of the Bohr model
28
New cards
octet rule
atoms are most stable when they contain eight electrons in their outermost shell
29
New cards
valence shell and number
the outermost shell of an atom
30
New cards
Products
molecule that is on the right side of a chemical equation
31
New cards
irreversible chemical reactions
\
when reactants in a chemical reaction form an irreversible product.
when reactants in a chemical reaction form an irreversible product.
32
New cards
cations
\
positive ion that is formed by an atom losing one or more electrons
positive ion that is formed by an atom losing one or more electrons
33
New cards
anions
\
always from -ide at the end
negative ion that is made by an atom gaining one or more electrons. Example: chloride (CL-)
always from -ide at the end
negative ion that is made by an atom gaining one or more electrons. Example: chloride (CL-)
34
New cards
Nonpolar covalent bonds
\
type of covalent bond where atoms share electrons between them equally
type of covalent bond where atoms share electrons between them equally
35
New cards
how to calculate pH
power of hydrogen (H+)
examples:
pH 7 is 1 times 10^-7 (means there are 7 OH- and 7 H+)
pH 3 is 1 times 10^-3 (means there are 11 OH- and 3 H+)
pH 12 is 1 times 10^-12 (means there are 2 OH- and 12 H+)
examples:
pH 7 is 1 times 10^-7 (means there are 7 OH- and 7 H+)
pH 3 is 1 times 10^-3 (means there are 11 OH- and 3 H+)
pH 12 is 1 times 10^-12 (means there are 2 OH- and 12 H+)
36
New cards
body
the amount of carbon in your ________ will stay the same as the atmosphere (homeostasis). will occur until you die, then carbon will turn back into nitrogen (which is how carbon dating works--→ you measure the amount of nitrogen in something)
N^14 in the atmosphere. Nitrogen usually is 7 electrons and protons. when c14 becomes n14, the unstable atom’s nucleus releases radiation
N^14 in the atmosphere. Nitrogen usually is 7 electrons and protons. when c14 becomes n14, the unstable atom’s nucleus releases radiation
37
New cards
polarity of water
needs to bond in a mickey mouse shape because of its uneven charge
oxygen is more electronegative than hydrogen= its more likely that a shared electron would be found near the oxygen nucleus than the hydrogen nucleus
oxygen is more electronegative than hydrogen= its more likely that a shared electron would be found near the oxygen nucleus than the hydrogen nucleus
38
New cards
high heat capacity
due to water’s hydrogen bonds
heat is energy and energy is a calorie
calorie- heat needed to raise the temp. of 1 g of water 1 degree
When using celsius, at 100 degrees to raise water
heat is energy and energy is a calorie
calorie- heat needed to raise the temp. of 1 g of water 1 degree
When using celsius, at 100 degrees to raise water
39
New cards
high kinetic energy
kinetic energy- movement energy
when water boils, molecules move and break their bonds
when water boils, molecules move and break their bonds
40
New cards
high heat of vaporization
due to water’s hydrogen bonds = greater temp. stability
you need more energy to raise temp. from 99 to 100 than you do to raise temp. from 32 to 33
the amount of energy needed to change one gram of a liquid substance to a gas
you need more energy to raise temp. from 99 to 100 than you do to raise temp. from 32 to 33
the amount of energy needed to change one gram of a liquid substance to a gas
41
New cards
hydrogen bonds
weak
42
New cards
cohesive
molecules in water are attracted to each other
surface tension creates miniscus
soap breaks surface tension
surface tension creates miniscus
soap breaks surface tension
43
New cards
adhesive
molecules in water are attracted to surfaces
molecules in water are more strongly attracted to the charged surface of something than they are to the other water molecules
molecules in water are more strongly attracted to the charged surface of something than they are to the other water molecules
44
New cards
capillary action
attraction of water molecules together causes movement
ex: drinking through a straw, spilling water onto a paper towel
ex: drinking through a straw, spilling water onto a paper towel
45
New cards
universal solvent
almost every substance will dissolve in water
hydrogen bonds allow ions and other polar molecules to dissolve
ex: salt in water
hydrogen bonds allow ions and other polar molecules to dissolve
ex: salt in water
46
New cards
Avogaders Number
one mole of water = 6 time 10^23 (lots of zeros)
47
New cards
low pH
lots of H+, less OH-
48
New cards
high pH
lots of OH-, less H+
49
New cards
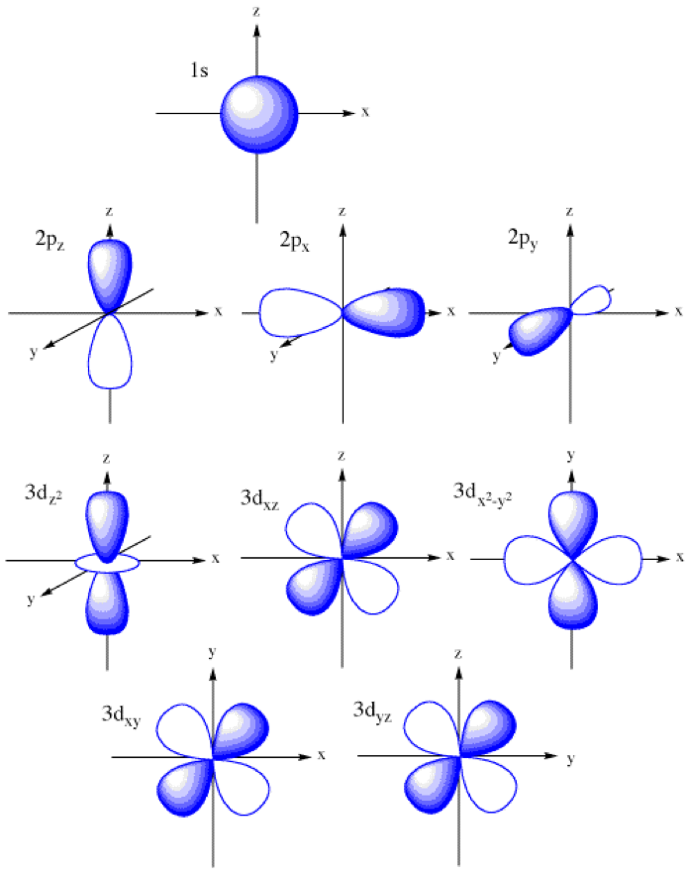
subshell shapes
50
New cards
chemical reaction
the ability to combine and to chemically bond with something else