Nuclear Chemistry Quiz
1/22
Earn XP
Description and Tags
Chemistry Unit 3
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
23 Terms
What is a nucleon ratio?
a ratio of protons to neutrons
How do neutrons help the atom?
they act as a glue and keep it stable
When is an atom considered unstable?
if the nucleon is greater than 1:1.5
What is the last non-radioactive element on the periodic table?
#82
What is chemical stability?
how resistant an element is when undergoing a chemical change (the electrons)
What is nuclear stability?
how likely a nucleas is to stay intact and resist decay (the protons and neutrons)
Alpha Particles
essentially a helium
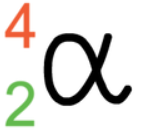
Beta Particles
an electron
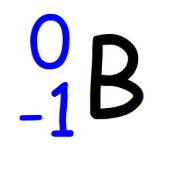
Gamma particle
like a photon or ray
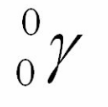
What is nuclear bombardment?
a manmade chemical reaction that essentially shoots a particle at a pre-existing nucleas
Neutron
energy released
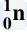
What does a Geiger counter do?
Measures radiation
What is nuclear fusion?
Combining two small massed elements
Pros to nuclear fusion
-low waste
-clean energy
-produces more energy
Cons to nuclear fusion
-really complicated
-really hard to do
What is nuclear fission?
Splitting large massed elements to collect their energy
Pros to nuclear fission
-easy
-efficient
Cons to nuclear fission
-produces lots of waste
-produces less energy
Where does nuclear fission occur?
In the dome building of a nuclear fission plant
How much U-235 is in a nuclear fission plant?
less than 1% of what was in the atomic bomb
What produces the energy in a nuclear fission plant?
the small controlled reaction creates steam which is converted to energy
T/F the smoke stack emits smoke in a nuclear fission plant?
False
How is steam converted to energy?
Through turbines and generators