Ch.7 Chemical Energy
1/43
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
44 Terms
Energy
is the capacity to do work (displace or move matter) or
to produce heat
Law of conservation of energy
energy can be converted from
one form to another but can be neither created nor destroyed
Total energy content of the universe is constant
Kinetic Energy
KE = ½mυ2
m = mass, kg
υ = velocity, ms–1
Units are
kg m2 s–2 J
Potential Energy
in a gravitational field, but also the composition of matter
PE = mgh
m = mass, kg
g = gravity, ms–2
h = height, m
Units are
kg m2 s–2 J
Work
is defined as the transfer of energy that occurs when a force is
applied to an object, causing it to move.
Heat
is the transfer of energy between a system and its surroundings
due to a difference in temperature. Heat flows from hotter to colder
objects and is not the same as temperature — it is energy in transit,
not energy stored.
Elastic Collision
objects bounce off
each other with no loss in total kinetic
energy, for example collisions
between gas molecules.
Inelastic Collision
some kinetic
energy is converted into other forms
(heat, sound, deformation). Example:
a car crash or a ball of clay hitting the
ground and sticking.
Higher energy systems are less stable
than the lower energy ones.
Heat
is energy transfer between a system and its surroundings,
caused by the temperature difference.
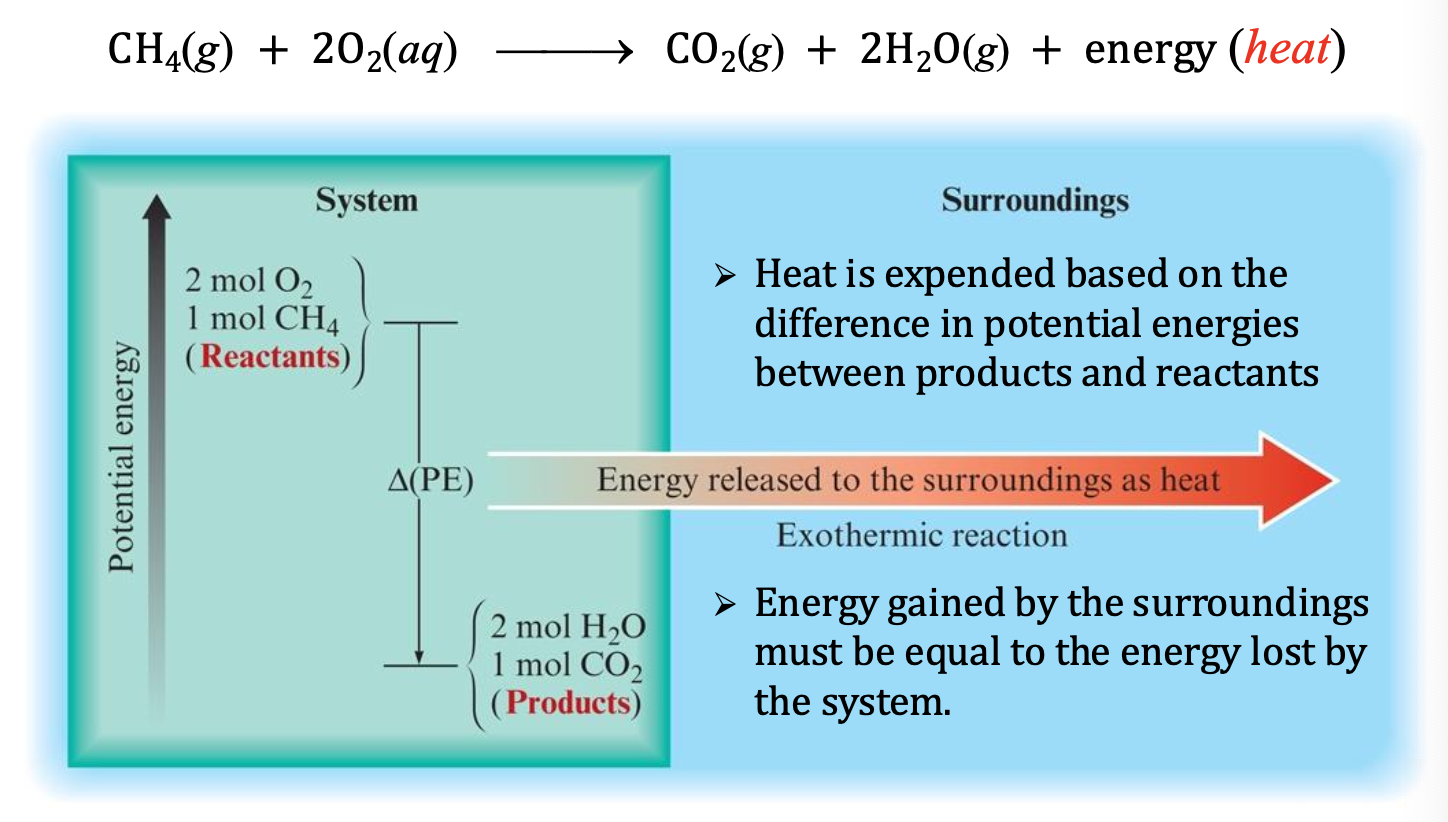
Exothermic Reaction
reaction gives off heat
❖ In an isolated system, system T increases
❖ In a non-isolated system, heat is given off to the surroundings,
i.e., qrxn < 0 (negative)
Reactants have more energy, products have lower energy
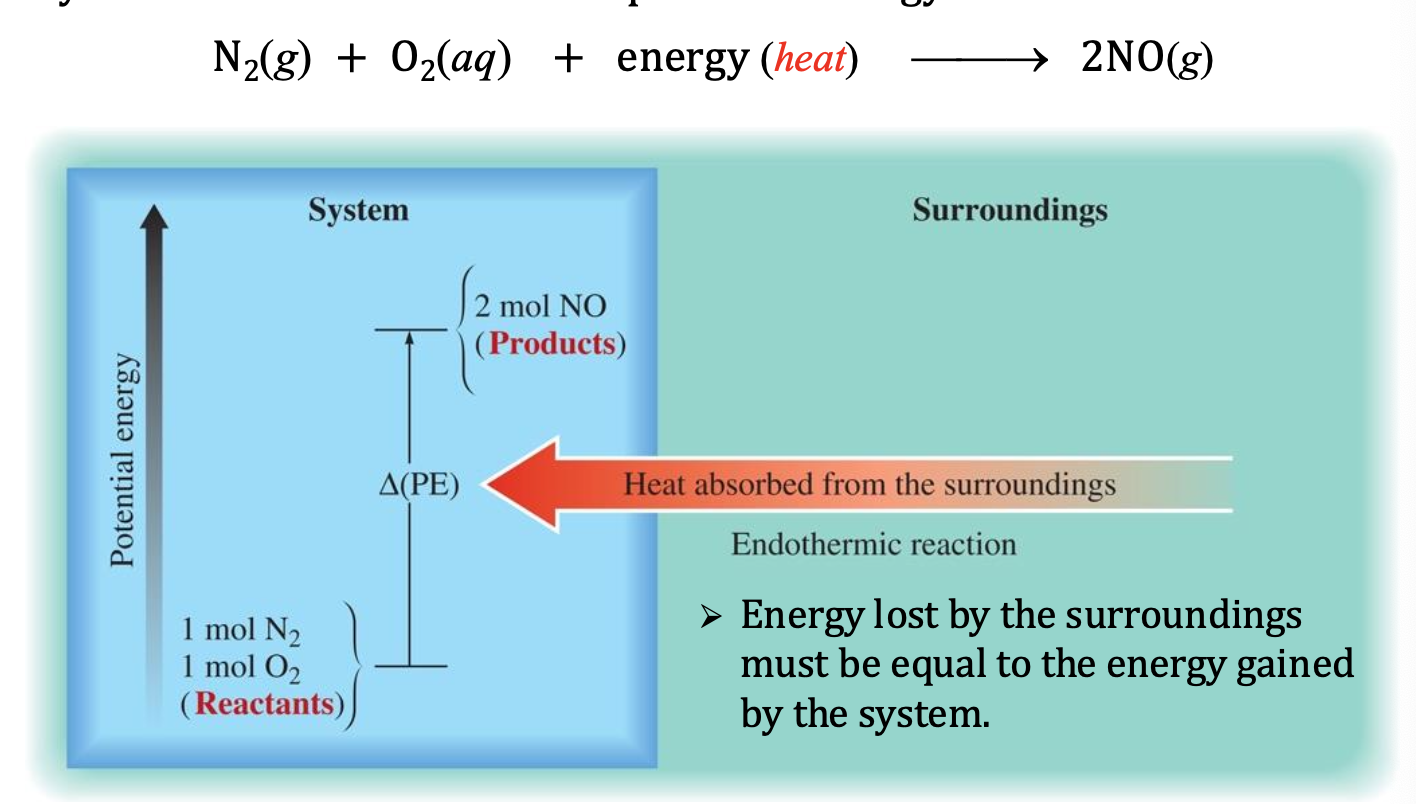
Endothermic Reaction
reaction absorbs heat
❖ In an isolated system, system T decreases
❖ In a non-isolated system, heat is absorbed from the
surroundings, i.e., qrxn > 0 (positive)
Reactants have lower energy, products have higher energy
Unlike heat, work is caused by a
force moving through a
distance (heat is caused by a temperature difference)
A negative quantity of work
signifies that the system loses
energy
A positive quantity of work
signifies that the system gains
energy
Chemical energy
energy due to chemical bonds and intermolecular forces
Thermal energy
translation, vibration, and rotation of molecules/atoms.
Heat (q):
Transfer of thermal energy due to a temperature difference.
Enthalpy (H):
A thermodynamic quantity related to heat at constant pressure:
ΔH=ΔE+PΔV
qrxn
is the quantity of heat exchanged between
a reaction system and its surroundings.
PV work (w)
Work (w) = − P ΔV
Enthalpy
is a state function—E, P, and V are all state functions;
therefore H must be a state function also
Positive values indicate
endothermic reactions - N2(g ) + O2(g ) ⎯→ 2NO(g ) ΔH = +180 kJ
Negative values indicate
exothermic reactions
State function (or state property)
is a property of the system that depends only on the current state of the system, any change in its value is independent of how the change in state was brought about
Work and heat are
NOT state functions
q < 0, w < 0
Energy leaving a system
carries a negative sign If heat given off by the
system
If work is done by the system,
Energy entering a system
carries
a positive sign:
If heat absorbed by the
system
q > 0
If work done on the
system
w > 0
Heat capacity (C)
of a substance is the quantity of heat required
to change the temperature of the substance by 1 ℃
C = q/ΔT (units are J/℃ or J/K)
Molar heat capacity, 𝐶𝑚
energy required to raise the
temperature of one mole of pure substance by 1℃
Specific heat capacity, 𝐶𝑠
energy required to raise the temperature of one gram of pure substance (or solution) by 1℃
𝐶𝑠 = C/m = q/(m× ΔT)
Constant-Pressure Calorimetry
−𝑞𝑟𝑥𝑛 = q calorimeter
= mass specific heat ΔT
𝑞𝑟𝑥𝑛 = −𝐶𝑐𝑎𝑙∆𝑇
Constant–Volume Calorimetry
− q rxn = q calorim = q V = ΔE
q calorim = C × ΔT
Petroleum & Natural Gas
Fossil fuels like gasoline and methane provide energy via combustion.
Nonrenewable resources contribute to CO₂ emissions and climate change.
· Air pollution: Burning fossil fuels releases CO₂, NOₓ, and particulates, contributing to smog and respiratory issues.
· Water pollution: Oil spills and wastewater from fracking contaminate ecosystems.
· Greenhouse gases: CO₂ and methane emissions from drilling and combustion contribute to climate change.
· Nonrenewable: These resources are finite, with extraction causing land degradation.
Effects of Carbon Dioxide on Climate
CO₂ traps infrared radiation, leading to global warming.
· Effects include melting ice caps, rising sea levels, and extreme weather patterns.
· Increased atmospheric CO₂ levels correlate with rising global temperatures over the past century.
Wind Energy
· Clean and renewable: No emissions during operation.
· Land use: farms can coexist with agriculture.
· Wildlife concern: Improperly placed turbines can impact bird and bat populations.
Hydrogen as a Fuel
Hydrogen + O₂ → electricity + water (via fuel cells).
Zero emissions at the point of use (only water vapor released).
· Production method matters: If made from natural gas ("gray hydrogen"), it still emits CO₂.
· Storage and transport: Require high energy and infrastructure.
Other Energy Alternatives
· Solar: No emissions during use, but panel production involves mining and energy use.
· Biofuels: Renewable but can compete with food supply and require land use changes.
· Geothermal: Minimal emissions, but can release trace gases and require water.
Coal
· High energy content but also high pollutant output (SO₂, mercury, CO₂).
· Environmental issues: acid rain, smog, and mining damage.
High CO₂ emissions: Coal has the highest carbon footprint among fossil fuels.
· Mining damage: Strip mining and mountaintop removal devastate landscapes and wildlife habitats.
· Acid rain: Sulfur dioxide (SO₂) released during burning reacts in the atmosphere to form acid rain, harming forests and aquatic systems.
· Ash waste: Disposal of coal ash can lead to contamination of water supplies.
Green Chemistry Principles:
o Minimize energy use (favor reactions that occur at room temperature and pressure).
o Reduce waste and avoid toxic by-products.
o Design processes that maximize atom economy and use renewable feedstocks.