Heat of neutralisation experiment
1/25
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
26 Terms
Heat of neutralisation is the heat change when 1 mole of H+ ions from an acid react with 1 mole of OH- ions from a base.
Define heat of neutralisation
good proton donor
will completely dissociate in water into their ions.
What are the characteristic properties of a strong acid?
H=m.c.t (V. IMPORTANT- MUST UNDERSTAND)
m= total mass of acid and base (in kg)
c= specific heat capacity of water
t= change in temperature
What is the formula for heat of neutralisation?
We measure out 30cm3 of 1M hydrochloric acid into a graduated cylinder.
What acid do we use in this experiment?
Polystyrene cup
What kind of cup do we use in this experiment?
We measure out 30cm3 of sodium hydroxide 1M into a graduated cylinder.
What base do we use in this experiment?
Polystyrene is a good insulator.
It minimises heat loss to the surroundings.
This allows for a more accurate temperature reading.
Why do we use a polystyrene cup and a lid?
Equal moles of HCL and NaOH were placed in the cup. If some acid or base splashes out of the cup, the recorded temperature change is not accurate.
Why do we need to avoid splashing in this experiment?
Neutralisation reactions are exothermic. The temperature on the thermometer increases but eventually falls due to heat loss to surroundings.
What type of reaction is a neutralisation reaction?
Ensures you get a larger, measurable temperature change (as the thermometer might not register temperature change in very dilute amounts of acid and base)
Why do we use more concentrated solutions of acid and base during this experiment?
Helps minimise heat loss to the surroundings.
Why do we quickly add the solutions and place a lid on the cup?
Get a more accurate result for temperature change.
Avoid taking temperature in a hot or cold spot.
Why do we stir the acid and the base when the two mixtures are added?
The temperature rise is still the same.
What happens if we use larger volumes of acid and base (but of the same concentration)?
Use a digital thermometer- provides a more accurate temperature reading.
Repeat several times and get an average value for the temperature change.
Use a lid/ use more insulation (eg wrap in cotton wool)
How could you improve this experiment’s accuracy?
Firstly, calculate the moles of HCL (on its own).
Next, get the mass of HCL and NaOH in kg
Place into formula H=m.c.t
Then adjust the heat for 1 mol (ie if you’ve calculate 0.02 moles of HCL, adjust to 1 mol)
Outline how we calculate the heat of neutralisation
The thermometer might not register temperature change in very dilute amounts of acid and base.
What would happen if you used very dilute amounts of acid and base?
Whilst more heat is produced due to a greater number of moles being neutralised, the heat will then be absorbed by a larger volume of solution.
Why will the temperature rise remain the same when larger volumes of acid and base (of the same concentration) are used?
The heat change when the number of moles of reactants according to a balanced equation react completely
Define heat of reaction
An exothermic reaction releases heat.
What is an exothermic reaction?
An endothermic reaction takes in heat.
What is an endothermic reaction?
The heat change when one mole of a substance is completely burned in excess oxygen.
Define heat of combustion
The burette is more accurate than a graduated cylinder.
Give an advantage of using a burette to measure out the base
The burette is slow. It allows for more heat loss to the surroundings.
Give a disadvantage of using a burette to measure out the base
This is an exothermic reaction.
Why did the temperature rise when the solutions were mixed?
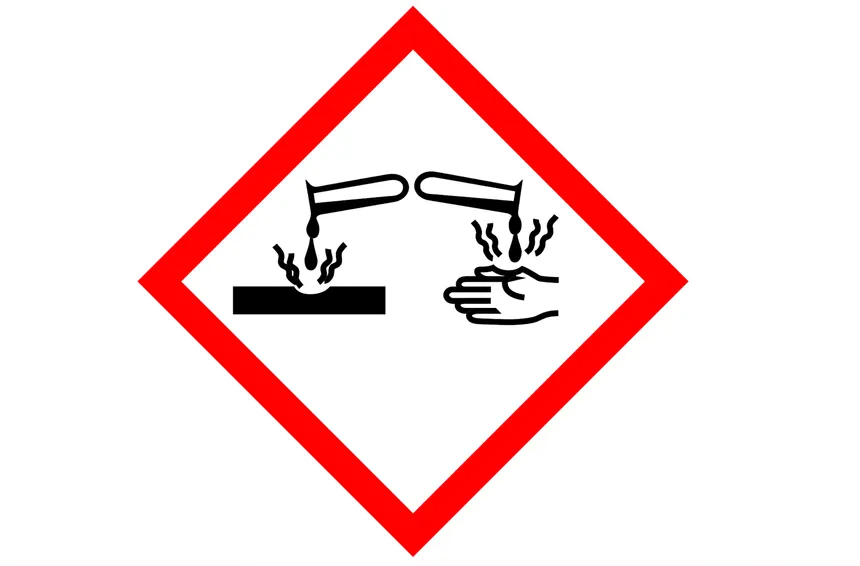
Liquid pouring from test tube onto hand (and metal object)
Draw and describe the hazard warning pictogram that should be used for NaOH.
Draw and describe the hazard warning symbol for hydrochloric acid