physics - radioactivity (6.1 - 6.46)
1/74
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
75 Terms
6.1 atom
positively charged nucleus
consists of protons & neutrons
contains almost all mass
radius much smaller than atom radius
surrounded by negatively charged electrons
6.2 typical size of atoms & small molecules
atom = 1 × 10-10m
molecule = 1 × 10-9m
6.3 structure of nuclei of isotopes
isotope: 2 atoms of same element with diff. numbers of neutrons
e.g. carbon-13:
proton number = 6: 6 protons
mass number = 13: 6 protons, 7 neutrons
number in name (13) = mass number
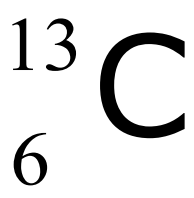
6.4 nucleus charge
characteristic positive charge
6.4 isotopes mass
isotopes of element differ in mass - diff. numbers of neutrons
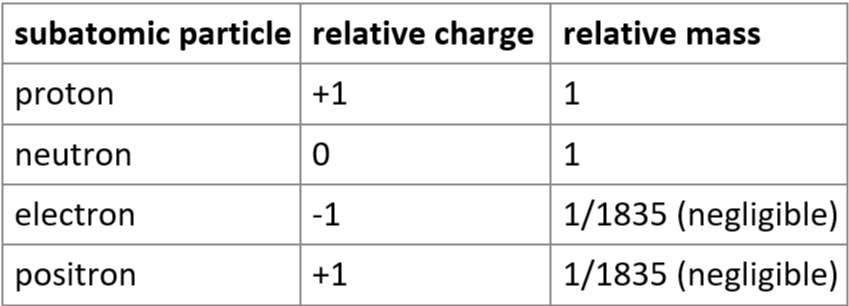
6.5 protons, neutrons, electrons, positrons - relative masses & relative electric charges
6.6 number of protons & electrons, charge of atom
number of protons = number of electrons - charge is neutral
6.7 electrons in atom
electrons orbit nucleus at diff. set distances from nucleus
6.8 what do electrons do when there’s absorption/emission of EM radiation?
electrons change orbit
atom absorbs EM radiation: electrons → higher orbit
atom emits EM radiation (can be visible light): electrons → lower orbit
6.9 how do atoms form positive ions?
atom gains energy - loses outer electrons
loses electron = protons > electrons = + charge = + ion
6.10 α, β-, β+, γ rays, neutron radiation - emitted from where & in what process? (general)
from unstable nuclei
in random process
6.11 α, β-, β+, γ rays - type of radiation (general)
ionising radiation
6.12 background radiation
weak, ionising radiation - constantly exposed to from space & naturally radioactive substances in environment
6.13 origins of background radiation - earth
radon:
main source
radioactive gas
produced by rocks containing small amount of uranium
diffuses into air from rocks & soil
can build up in houses - esp. where poor ventilation
amount in air depends on type of rock & its uranium content
some food:
naturally contain small amounts of radioactive substances
hospital treatments:
e.g. X-rays, gamma-ray scans, cancer treatments
6.13 origins of background radiation - space
sun & other stars:
high-energy, charged particles (cosmic rays) stream out of them - form of radiation
many cosmic rays stopped in atmosphere
some still reach earth’s surface
6.14 measuring & detecting radioactivity - photographic film
more radiation reaches it - becomes darker
film must be developed to measure amount of radiation (dose)
(some dosimeters (film badges) change colour without needing to be developed)
6.14 measuring & detecting radioactivity - Geiger-Muller tube
radiation passes through tube - ionises gas inside, lets short pulse of current flow
connected to counter - counts pulses of current
clicks each time radiation detected
count rate = number clicks/sec or min
6.15 what is an α particle equivalent to?
helium nucleus
2 protons, 2 neutrons
relative mass = 4
charge = +2
6.15 what is β- particle?
electron
relative mass = 0
charge = -1
what is a β+ particle?
proton
relative mass = 0
charge = +1
6.15 where is β particle emitted from?
nucleus
6.15 what is gamma ray?
EM radiation
(high frequency)
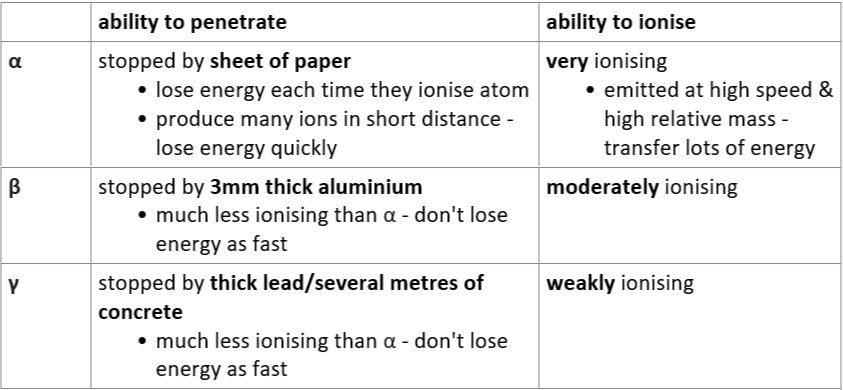
6.16 α, β, γ - ability to penetrate & ionise
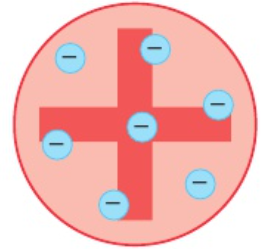
6.17 Thompson’s plum pudding model
atoms contain electrons (- charge, very small mass)
atom = + ‘pudding’
- electrons = ‘plums’ scattered through ‘pudding’
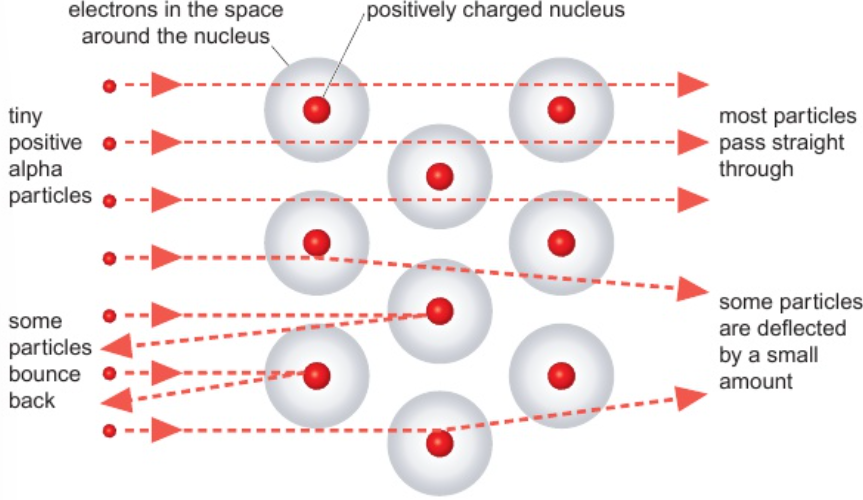
6.17 Rutherford α particle scattering
what happens when + α particles passed through gold foil
most α particles passed through gold foil, few bounced back - plum pudding model can’t explain
atoms mostly empty space, most mass in tiny central nucleus (+, electrons moving around it)
6.17 Bohr model
amended Rutherford’s atomic model to explain atoms absorbing & emitting light
electrons in certain fixed orbits (shells) around nucleus
cannot be part way between 2 orbits
explains lines in emission & absorption spectra
6.18 β- decay
neutron → proton + electron
electron (β particle) ejected from atom
atomic number: +1
mass number: no change
6.19 β+ decay
proton → neutron + positron
positron (β particle) ejected from atom
atomic number: -1
mass number: no change
6.20 α decay - effect on atomic number & mass number
atomic number: -2
mass number: -4
6.20 β- decay - effect on atomic number & mass number
atomic number: +1
mass number: no change
6.20 β+ decay - effect on atomic number & mass number
atomic number: -1
mass number: no change
6.20 γ decay - effect on atomic number & mass number
atomic number: no change
mass number: no change
neutron emission
neutron emitted
6.20 neutron emission - effect on atomic number & mass number
atomic number: no change
mass number: -1
6.21 what happens to nuclei that have undergone radioactive decay?
nuclear rearrangement
lose energy as gamma radiation
α, β-, β+, neutron - symbols
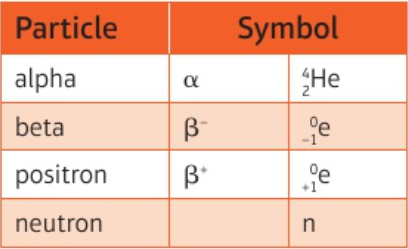
6.22 use given data to balance nuclear equations - mass & charge

6.23 how does activity of radioactive source decrease over time?
nucleus decays - becomes more stable
sample of substance contains more stable nuclei = lower activity
activity definition
number of nuclear decays/second
6.24 unit of activity of radioactive isotope
Becquerel (Bq) (= 1 nuclear decay/second)
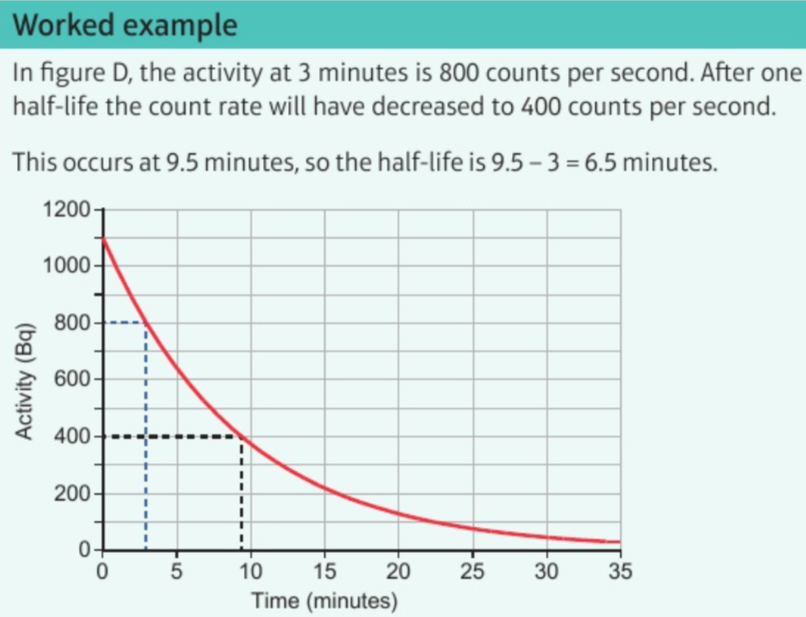
6.25 half-life of radioactive isotope
time taken for half undecayed nuclei to decay
or time taken for activity of source to decay by half
6.26 predicting decay & half-life
cannot predict when particular nucleus will decay - decay is random process
half-life lets us predict activity of large number of nuclei during decay process
6.27 calculations on decay of radioactive isotope using half-life
6.28 uses of radioactivity - household fire (smoke) alarms
smoke alarm contains source of α particles
detector has electrical circuit with air gap between 2 electrically charged plates
α particles released - ionise molecules in air
ions attracted to plates with opposite charge - let small electrical current flow
current flowing - alarm won’t sound
smoke gets into air gap → smoke particles slow down ions → current flowing across gap decreases
current drops below certain level - alarm sounds
6.28 uses of radioactivity - irradiating food
foods contain microorganisms - cause decomposition
irradiated with gamma rays - kill bacteria
food: safer to eat; stored longer
doesn’t make food radioactive
6.28 uses of radioactivity - sterilising equipment
some instruments (e.g. plastic syringes) can’t be heated to kill microorganisms
sealed in bags, irradiated with gamma rays - penetrate bag & equipment
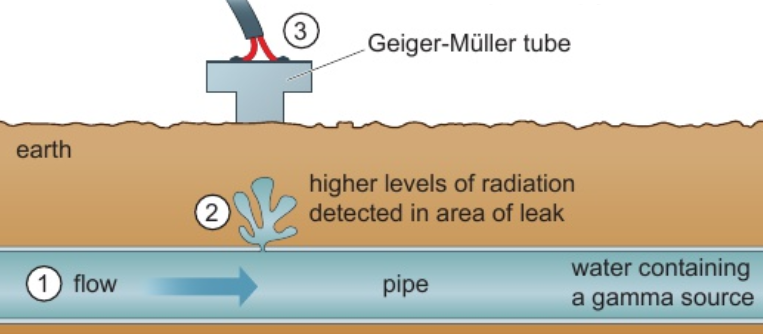
6.28 uses of radioactivity - tracing
radioactive isotopes used as tracers
e.g. gamma source added to water - detects leaks in water pipes underground
at leak water flows into surrounding earth
GM tube follows path of pipe - detects more radiation at leak
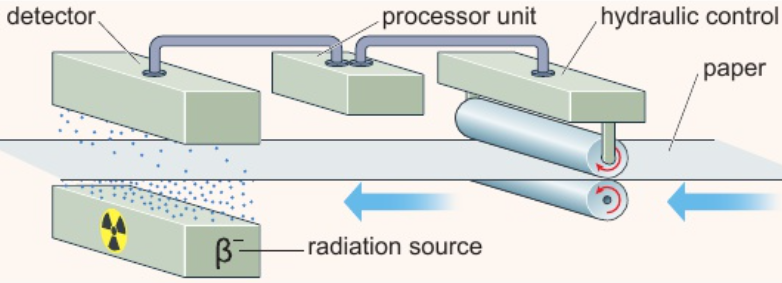
6.28 uses of radioactivity - gauging thicknesses
2 rollers squeeze wood pulp with force - produces diff. paper thicknesses
detector counts rate β particles get through paper from source on one side
paper too thin: more β particles penetrate paper → detector records higher count rate → computer senses higher count rate → reduces force on rollers → paper thicker
paper too thick: less β particles penetrate paper → detector records lower count rate → computer senses lower count rate → increases force on rollers → paper thinner
6.28 uses of radioactivity - diagnosing & treating cancer
diagnoses cancer: tracers in body
treats cancer
6.29 dangers of ionising radiation (body)
large amount: tissue damage
small amounts over long period of time: mutations (damages DNA in cell)
make cell malfunction
may cause cancer
can be passed onto next gen.
not all mutations harmful
cells often repair damage if dose is low
6.29 dangers of ionising radiation - precautions
sources handled with tongs - distance from source increases = intensity of radiation decreases
sources stored in lead-lined containers
6.30 how do dangers of ionising radiation depend on half-life?
contaminated with radioactive materials with longer half-lives poses greater hazard
effects last longer than for materials with shorter half-lives
6.31 precautions to ensure safety of people exposed to radiation - liming dose for patients
only exposed to radiation when benefits are greater than possible harm radiation could cause
minimum possible dose used
sources with short half-lives used - minimises time patient is exposed
6.31 precautions to ensure safety of people exposed to radiation - risks to medical personnel
increase distance from source
shield source
minimise time spent in presence of sources
exposure monitored using dosimeter badges
6.32 contamination
radioactive material particles on skin/in body
exposed to radiation as unstable isotopes in material decay
until material all decayed/source of contamination removed (not always possible)
water & soils contaminated - spreads into food chain
6.32 irradiation
exposed to α/β/γ radiation from nearby radioactive materials
person moves away - radiation stops
could expose cells to damage & mutation
6.33 treating tumours - radiation applied internally
internal radiotherapy:
β emitter (e.g. iodine-131) placed in/close to tumour
doesn’t always require surgery - patient stays in room alone while source in place
6.33 treating tumours - radiation applied externally
external radiotherapy:
beams of γ rays/x-rays/protons directed at tumour from outside body
several weaker beams directed at tumour from diff. directions - only tumour absorbs lots of energy & surrounding tissues harmed as little as possible
6.34 uses of radioactive substances in diagnosing medical conditions - PET scanners
tracer emits positron
positron meets electron - both destroyed, 2 gamma rays emitted in opposite directions
detector in PET scanner moves around patient - produces images showing where diff. amounts of γ radiation come from
6.34 uses of radioactive substances in diagnosing medical conditions - tracers
tracers contain radioactive isotope attached to molecules - taken up by particular organs
radioactive tracer emits γ rays
γ cameras detect location of tracer in body
find source of internal bleeding:
tracer injected into blood
γ cameras detect area of highest γ radiation - location of bleeding
detect tumour:
tracer made using radioactive glucose molecules
tracer injected into blood
active cells (cancer cells) take glucose up more quickly than other cells
γ cameras detect area of highest γ radiation - location of tumour
6.35 isotopes used in PET scanners - produced nearby
radioactive isotopes used in medical tracers need short half-life - other parts of body affected as little as possible
lose their radioactivity quickly - must be made close to hospital
6.36 nuclear power generating electricity - advantages
don’t produce CO2: nuclear fuels don’t burn; don’t contribute to climate change
6.36 nuclear power generating electricity - disadvantages
major accidents could occur: very serious consequences for many people
negative public view: many don’t think benefits of nuclear energy are worth risks
expensive waste disposal: produce waste that will stay radioactive for millions of years; needs to be sealed into concrete/glass & buried safely
expensive decommission: parts become radioactive during use
6.37 what are nuclear reactions source of?
source of energy (fission, fusion & radioactive decay)
6.38 fission of U-235
U-235 nucleus absorbs neutron
splits into 2 smaller daughter nuclei (also radioactive)
emits 2/more neutrons
energy transferred by heating
6.39 controlled nuclear chain reaction
neutrons released absorbed by other nuclei (of same isotope)
nuclei become unstable & split - release more neutrons
chain reaction controlled if other materials absorb some neutrons
6.40 chain reaction controlled in nuclear reactor - moderator
fission reactions occur - neutrons leave fuel rods at high speed
fuel rods in holes in moderator
slows down neutrons - increases chance of absorption by another U-235 nucleus
6.40 chain reaction controlled in nuclear reactor - control rods
contain elements that absorb neutrons
placed between fuel rods in reactor core
to increase rate of fission: control rods moved out of core → fewer neutrons absorbed
to decrease rate of fission: control rods moved into core → more neutrons absorbed
6.41 how is thermal energy from chain reaction used to generate electricity in nuclear power station?
energy released from core transferred to coolant
coolant: pumped through reactor; water/gas/liquid metal
hot coolant pumped to heat exchanger - used to make steam
steam drives turbine → turns generator → produces electricity
6.42 products of nuclear fission (general quality)
radioactive
6.43 nuclear fusion
2 small nuclei combine to form larger nucleus
mass of new nucleus < total masses of 2 smaller nuclei
lost mass converted to energy - released
energy source for stars (hydrogen nuclei combine to form helium nucleus)
6.44 nuclear fusion vs nuclear fission
energy: fusion = more; fission = less
products: fusion = not radioactive; fission = radioactive
materials that contain fusion reactions become radioactive
disposing radioactive waste: fusion reactors - fewer problems; fission reactors - more problems
6.45 nuclear fusion - high temp.
nuclei more likely to collide at higher temps. - moving faster
nuclei fast enough - overcome electrostatic repulsion & fuse
6.45 nuclear fusion - high pressure
for nuclei to fuse, must be close together
+ protons in nuclei repel (electrostatic repulsion of protons)
nuclei close enough - overcome electrostatic repulsion & fuse
6.46 conditions for fusion & making power station
fusion requires extreme temps. & pressures - hard to sustain
difficult to be practical & economic power station