EXAM 2 PATHOLOGY 1
1/93
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
94 Terms
Hypersensitivity Reactions are…
An excessive immune response that leads to harmful host reactions rather than protection but requires a pre-sensitized state of the host!
Explain Type I Hypersensitivity
IgE mediated: Pre-exposure to antigen causes production of IgE and binding to macrophages (sensitizing). When IgE encounters antigen again it produces massive degranulation of mast cells
Happens in atopic individuals
IL-4, IL-5, IL-10 and TNF release
Can cause local reactions or systemic reactions ( anaphylactic shock)
Explain Type II Hypersensitivity → examples
IgG or IgM antibodies bind to intrinsic or extrinsic antigens
Causes cytotoxic or non-cytotoxic reactions
Activates complement, ADCC, Opsonization, Phagocytosis, Antibody Mediated Cellular destruction
Examples:
Myasthenia gravis: antibodies bind to Ach receptors and prevent binding of Ach
Grave’s disease: antibodies bind to TSH receptors and cause excessive production of thyroid hormones
Erythroblastosis fetalis: rh- mom has rh+ fetus and produces IgG antibodies against it, then in second pregnancy it can cause the baby to have hemolytic anemia
Explain Type III Hypersensitivity → examples
Antibody-Antigen complexes forms in circulation or at sites of antigen deposition and becomes stuck
Causes complement activation and inflammation
Examples:
Systemic lupus erythematosus
Explain Type IV Hypersensitivity → examples
Not antibody mediated, it is T cell mediated by sensitized cells to environmental chemicals or persistent microbes
Can be CD4+ mediated or CD8+ mediated
Contact dermatitis, tuberculin test
Type I diabetes: CD8+ cells kill pancreatic islet Beta cells
Hashimoto thyroiditis: CD8+ cells kill thyroid epithelial cells
Types of grafts/transplants
Autograft: tissue or organ is transferred from one part of the body to another in the same individual
Isograft: tissue/organ transferred from one identical twin to the other
Allograft: tissue/organ transferred from one individual to another on the same species with some differences in HLA
Xenograft: tissue/organ is transferred between different species
Classification of Graft rejection
Timeframe
How does it happen
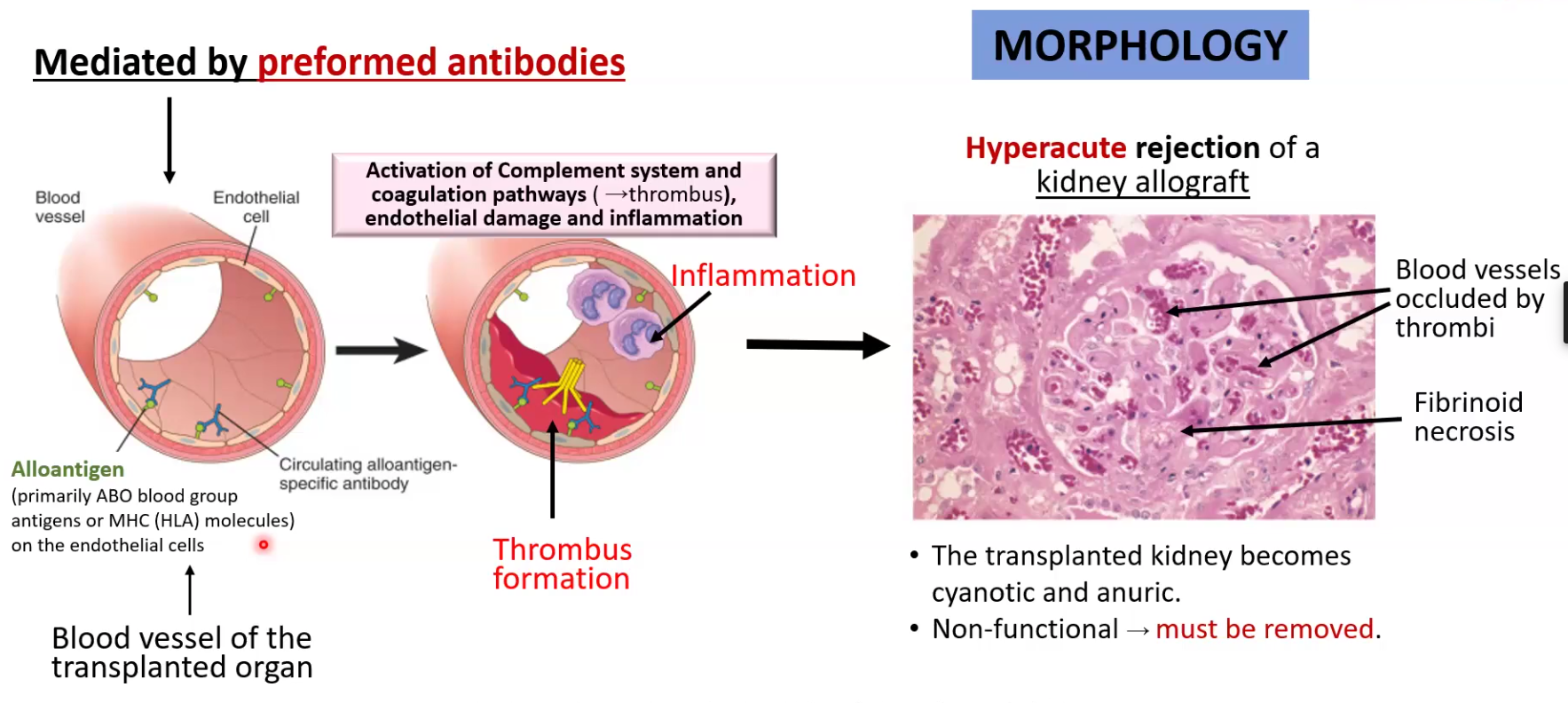
Hyperacute rejections
Happens minutes to hours after transplantation
Pre-formed antibodies bind to alloantigen and activate complement as well as thrombus formation
Transplanted organ becomes necrotic and must be removed
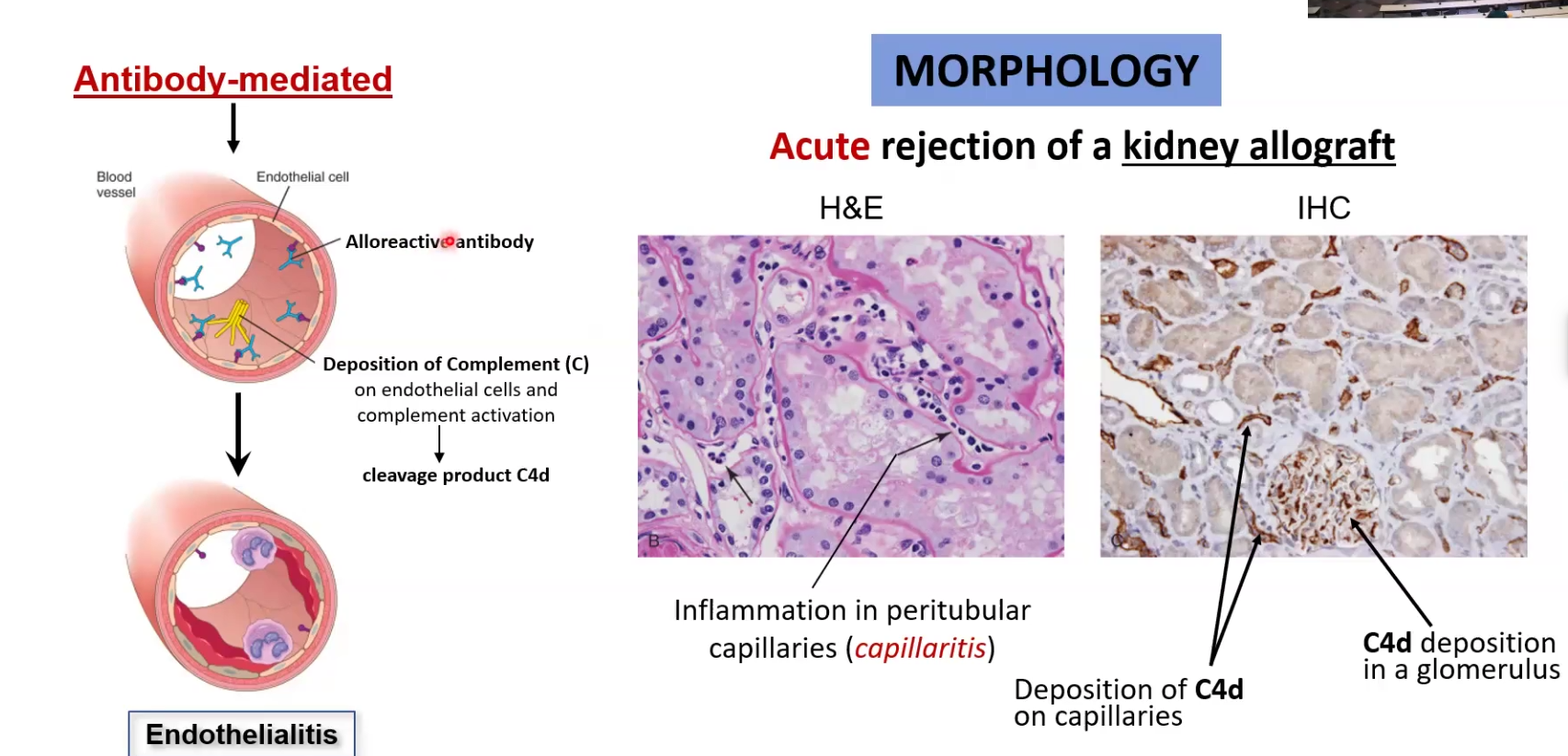
Acute rejections:
Happens in the first weeks or months
Can be mediated by T cells or antibodies
CD8 does direct killing while CD4+ T cells influence M and Neutrophils to kill
Antibodies are created after a while and activate complement
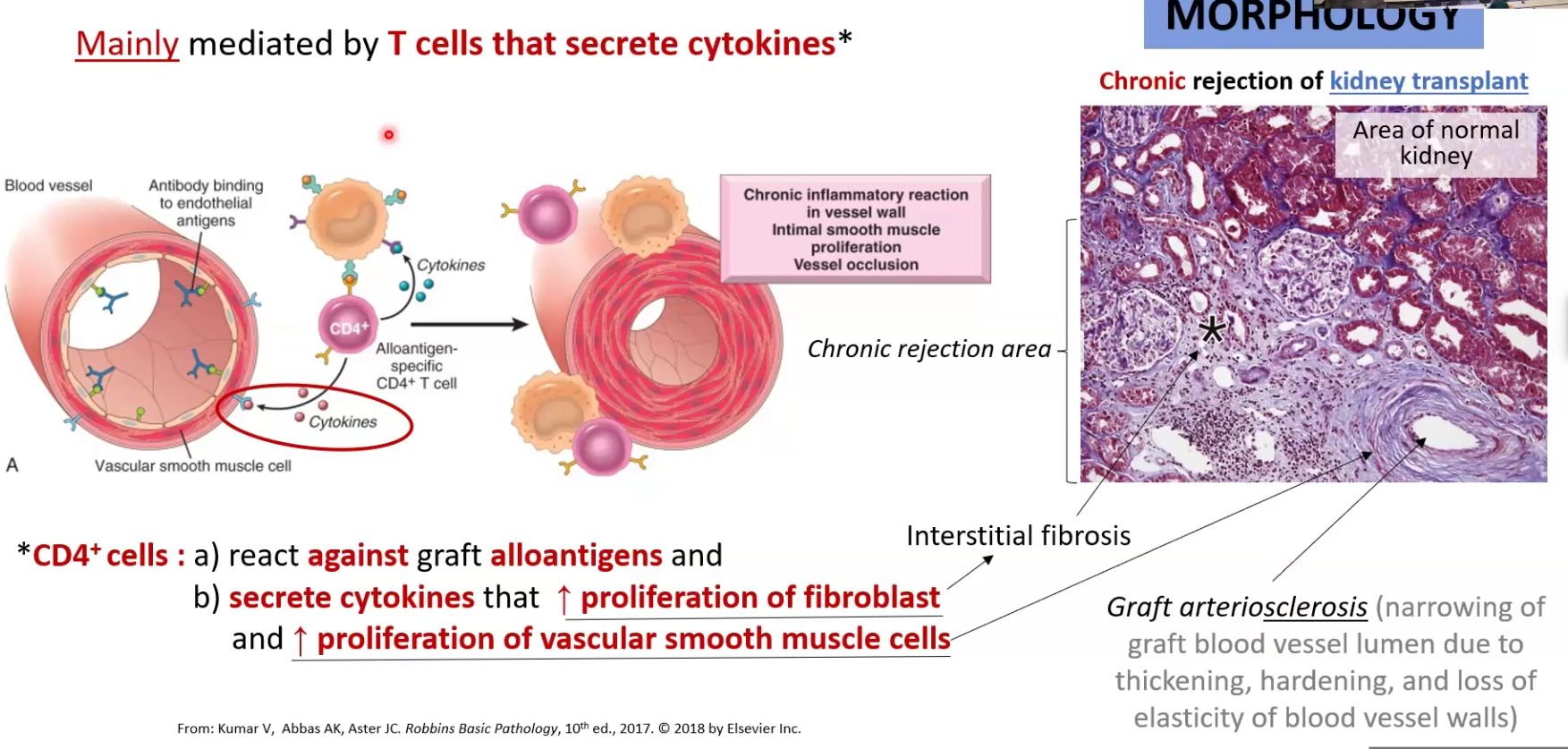
Chronic rejection:
Happens months or years later
Mostly CD4+ mediated: find alloantigen and release cytokines that stimulate fibroblast proliferation (causes fibrosis) and vascular smooth muscle cells (causes vessel occlusion)
Antigens can be involved as well
How do we prevent and Treat Allograft rejection
Prior:
Cross-Match Testing: confirm that ABO blood group of the donor and recipient are compatible
Tissue typing: compares HLA allele matching between donor and recipient
After:
Cyclosporine: suppresses T cell mediated immunity
Azathioprine: antiproliferative agent
Prednisone: anti-inflammatory steroid
Bone Marrow (BM) Transplantation
Used for
What must happen before BM grafting
Used for patients with bone marrow failure syndromes, inherited blood disorders, immunodeficiencies or hematologic malignancies
CAN RESULT FROM DEFECTIVE BM, OR DAMAGED BM BY CHEMO OR RADIATION
Before transplant, the host must be immunologically suppressed
Autologous vs Allogenic Transplant
Autologous: BM is collected from host, processed, cryopreserved, host undergoes chemotherapy, then patient is infused with BM
Allogenic: BM collected from donor, processed, host gets chemo, then BM is transplanted
Graft vs Host Disease (GVHD)
When does it occur
What cells cause it
2 types
Occurs following allogenic BM transplants
Caused by CD4 and CD8 cells in the graft that attack host tissues
Types:
Acute affects liver skin and GI
Chronic causes fibrosis and atrophy in one of the previous organs AND the lungs
What are the factors associated with autoimmunity? Explain Infection
Genetic susceptibility, Infections, Environmental insults such as UV radiation and smoking, gender (more frequent in women)
Infections: cells that are supposed to not be activated are because the pathogen induced B7 expression on APC and activates T cell. Another way is that the pathogen can do molecular mimicry and cause a reaction to self
Systemic Lupus Erythematosus
What kind of disease
Pathogenesis
What kind of antibodies have diagnostic value
Higher incidence in
Chronic systemic autoimmune disease
The person will have susceptibility genes that may become activated due to encreased burden of nuclear antigens, which will cause formation of anti-nuclear antibody complexes, the b cells will detect them and create more antinuclear antibodies → BUT CAN ALSO BE ANTI PHOSPHOLIPID
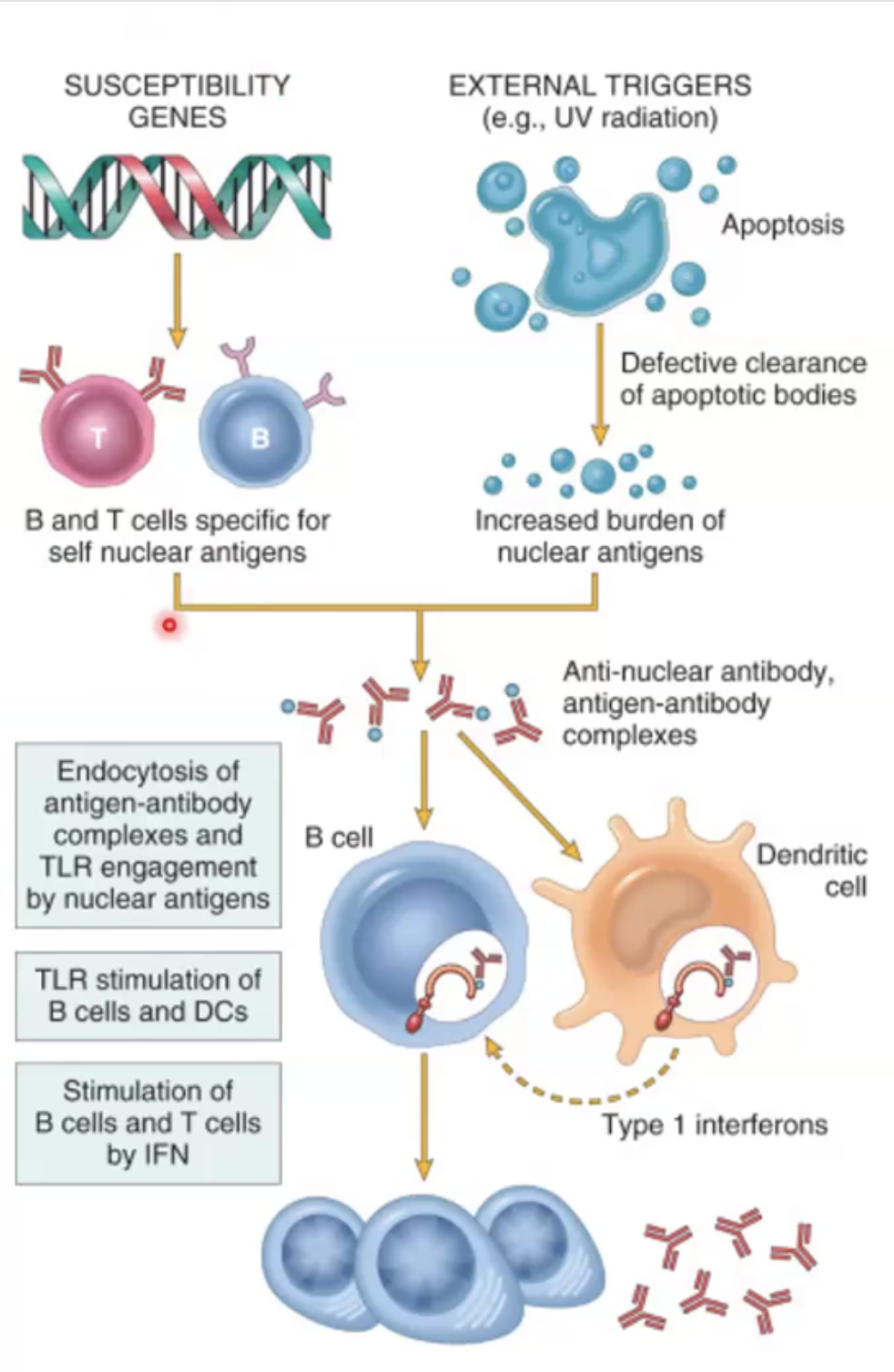
Anti dsDNA antibodies are associated with disease activity, prognosis and glomerulonephritis; Anti-smith antibodies are against RNP
Higher incidence in females of childbearing age and in hispanics/african americans
Criteria for Diagnosis of Systemic Lupus Erythematosus
Skin: malar butterfly rash, discoid rash, photosensitivity
Mucosa: oral or nasopharyngeal ulcers
Serositis: pleuritis and pericarditis
Joints: arthritis is 2 or more joints
Kidney: REnal disorders (75% of cases
CNS: neurological disorder
Blood: hematologic disorders
Antibodies: high titers of anto-dsDNA or anti smith antibodies in serum
Skin and Renal Involvement of SLE
Skin
Vacuolar degeneration of basal layer
Edema and perivascular inflammation
Vasculitis with fibrinoid necrosis
Renal
Deposition of immune complexes in the kidney
Sjogren Syndrome (SS)
What is it
Higher incidence in
Clinical Manifestations and what kind of hypers
Pathogenesis
Diagnostic criteria
An autoimmune disease that destroys lacrimal and salivary glands
50-60 yo females
Clinical Manifestations: Dry eyes, xerostomia, enlarged salivary glands, fibrosis and replacement by fat, hyperplasia obstructing ducts → Type IV hypersensitivity
Pathogenesis:
CD4 T cells and B cells infiltrate lacrimal and salivary glands
Rheumatoid factor (anti IgG)
Diagnostic criteria:
Schirmer Test: test tear production
Rheumatoid Factor
Lymphocytic infiltration in salivary glands
Systemic Sclerosis
Incidence is higher in
Pathogenesis
Limited Systemic vs Diffuse
Incidence is higher in females 25-50
Pathogenesis: unknown etiology but it is believed genetic susceptibility and external stimuli may cause abnormal production of cytokines that increase synthesis of ECM and cause fibrosis due to excessive collagen production
Limited : Skin involvement is confined to hands and face and visceral involvement doesn’t occur until late
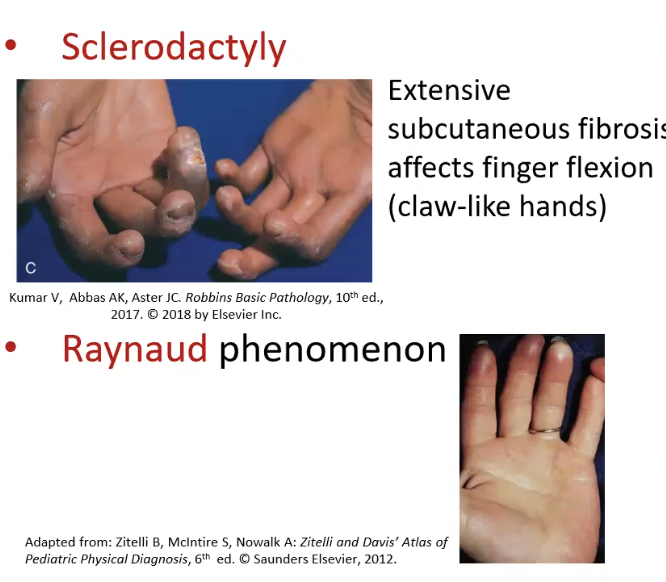
CREST syndrome: calcinosis, raynaud phenomenon, esophageal dysfunction, sclerodactyly, telangiectasis
Anti centromere antibodies
Diffuse: widespread skin involvement with early visceral involvement
antibodies against DNA topoisomerase
Bruton Disease (Agammaglobulinemia)
Primary or secondary immunodeficiency
Pathogenesis
Clinical Features
Primary
X-linked chromosome has a mutation in Btk gene that makes B cells unable to mature, therefore you have complete lack of Ig other than IgM
Recurrent infections due to lack of antibodies
DiGeorge Syndrome (Thymic Hypoplasia)
Primary or secondary immunodeficiency
Pathogenesis
Clinical Features
Primary
Gene deletion causes defective embryologic development of 3rd and 4th pharyngeal pouch → thymus is underdeveloped and unavle to activate T cells
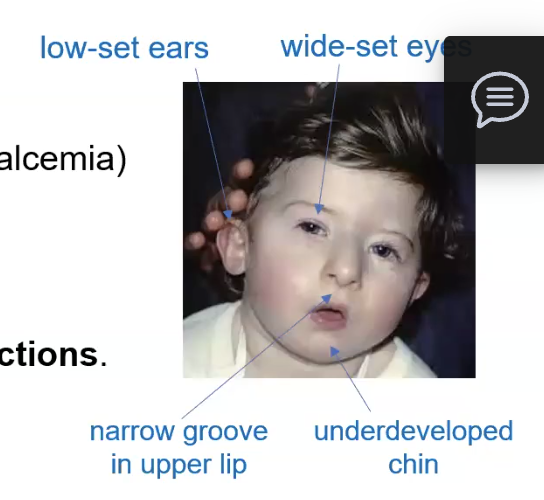
Hypoparathyroidism, Facial abnormalities, cleft palate, heart defects, susceptible to viral and fungal infections
Severe Combined Immunodeficiency (SCID)
Primary or secondary immunodeficiency
X-linked vs Autosomal recessive Pathogenesis
Treatment?
Primary
X linked: caused by mutation in IL2 receptor causes several IL to be absent, lack of IL-7 leads to absent expansion and proliferation of T AND B cells
Autosomal Recessive: caused by mutations in adenosine deaminase gene, which then causes buildup of ATP metabolites and inhibits DNA synth, which is toxic to lymhocytes
This type shows the lowest counts of T cell, Nk, B cells in SCIF
Treatment is Bone marrow transplant and sterile isolation because they are extremely susceptible to infections
Wiskott-Aldrich Syndrome (WAS)
Primary or secondary immunodeficiency
Pathogenesis
Clinical Features
Primary
Mutation of WAS gene in X chromosome (recessive) (almost always males) cause progressive loss of T cells in blood but they are fine in BM
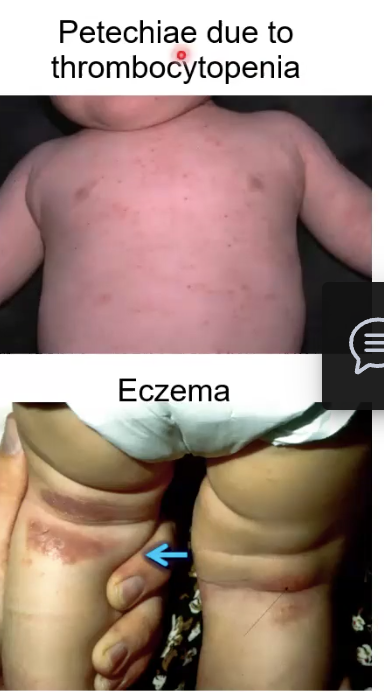
Recurrent infections, thrombocytopenia, eczema, petechiae
HIV
Primary or Secondary
Transmission
Important components of virus
Two major targets of HIV
Secondary
Secual, parenteral (blood), vertical (mother to infant)
Two copies of viral RNA, reverse transcriptase, gp120, gp41, integrase
CD4 T cells and MAcrophages
Pathogenesis of HIV
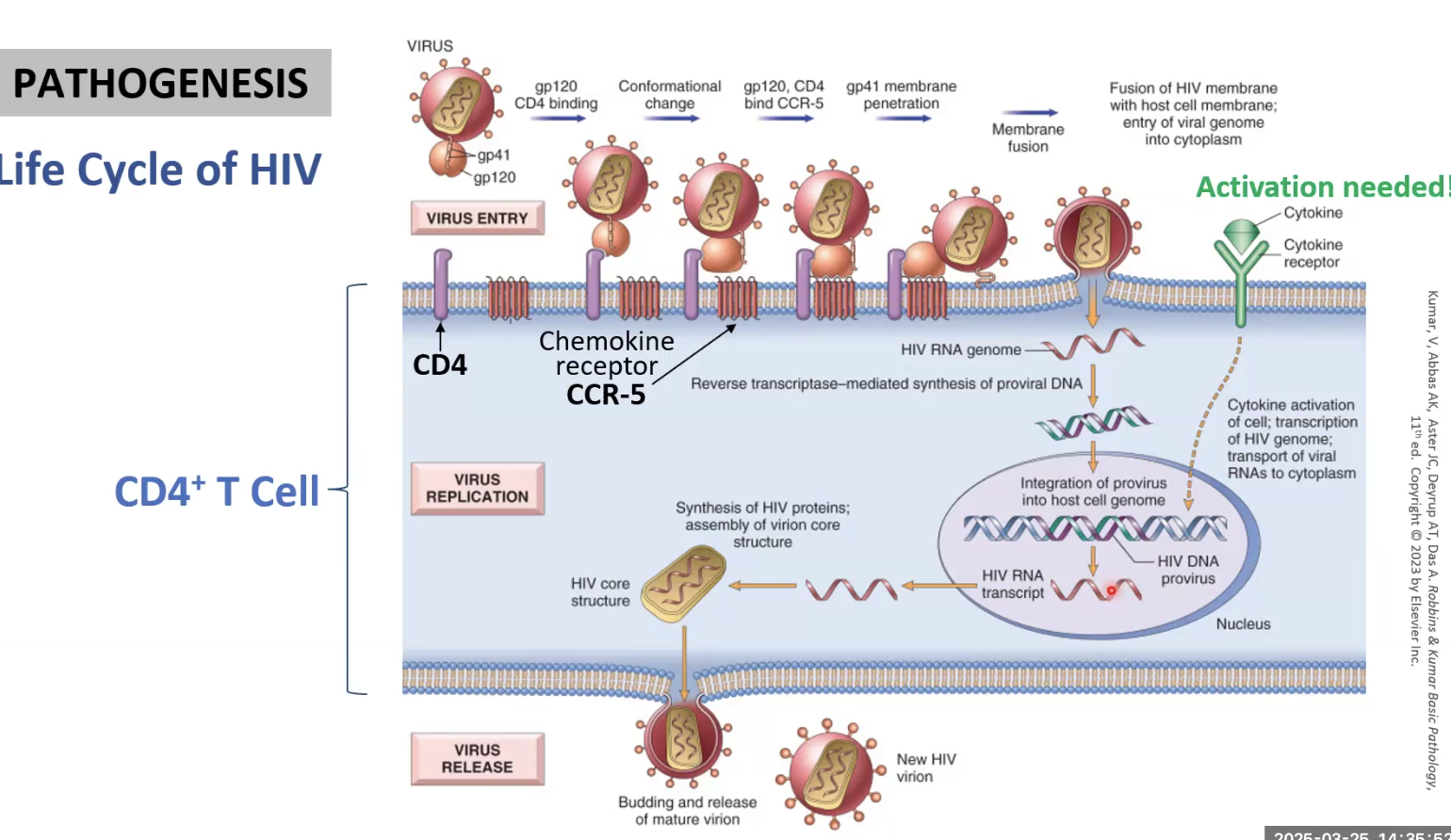
gp120 of HIV binds CD4 cell through its CXCR4 receptor
Conformational change allows CXCR4-gp120 complex to bind CCR-5 on CD4 cell
gp41 is able to penetrate, HIV enters cell
Cytokine activation needed !!!!
HIV integrates into DNA and then replicates itself using the host machinery
What happens to infected CD4 T cells?
How do macrophages become reservoirs of infection?
Infected CD4 T cells apoptose or are killed by CD8 T cells
Macrophages become infected bt they are resistant to CD8 T cells so they accumulate in tissue
Describe Phases of HIV Infection
Acute Phase: non-specific symptoms, viremia increases but CD8 cells attack and bring it down → 3-6 weeks after infection
Chronic: asymptomatic phase but may have flu-like symptoms or lymphoadenopathy, virus production low because T cells are keeping it in check→ can last up to 10 years
Crisis phase: CD$ T cell count drops below 200 and immune system severely compromised, serious risk of opportunistic infections, AIDS dementia, survival is only 3 years without treatment
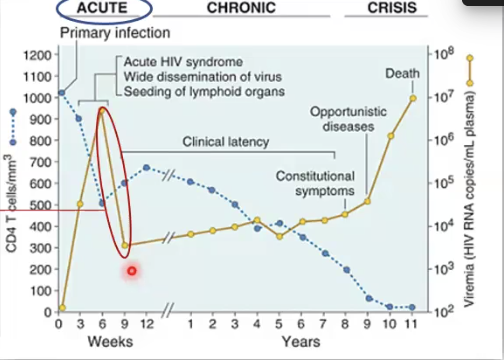
What are some common infections AIDS patients acquire?
Candidiasis, Pneumonia, CNS infections
Burkitt Lymphoma
Cervical and anal carcinomas
Kaposi’s sarcoma (vascular tumors) → herpes
Diagnosis of HIV
ELISA: colormetric immunosorbent assay thatputs HIV antigens with anti-HIV antibodies
Used as initial screening test
Western Blot: used to detect major HIV proteins such as gp120, gp24, gp41
Confirmatory test
Viral Load Test (RT-PCR): measures number of HIV RNA copies per mL of blood
Monitoring test to predict rate of decline of CD4 cells and progression of diseasd
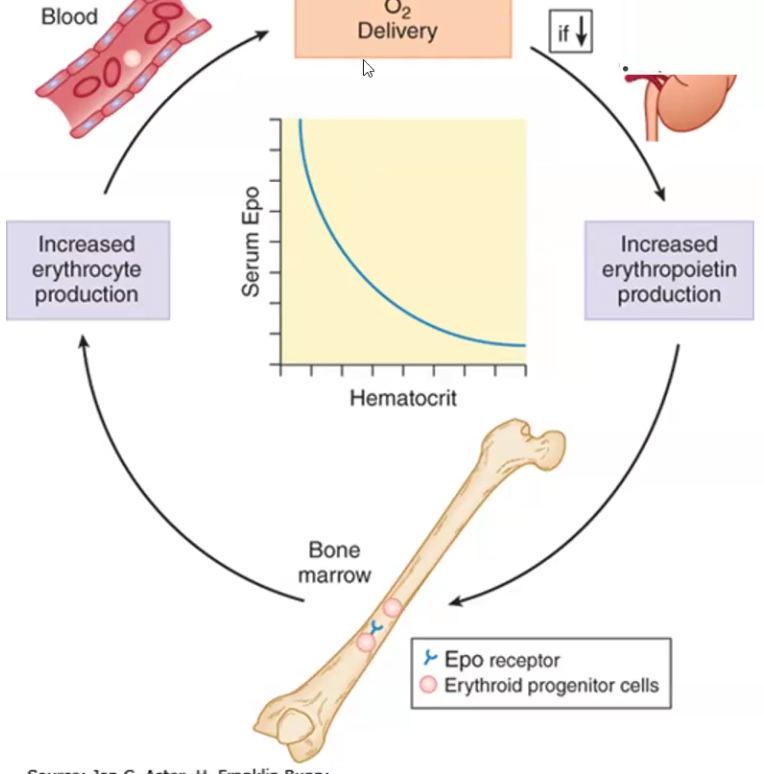
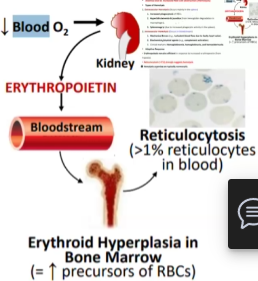
Hypoxia (decreased oxygen delivery) to specialized cells in the kidney results in increased expression and secretion of _________, which in turn increases ___ ____ ________
Hypoxia (decreased oxygen delivery) to specialized cells in the kidney results in increased expression and secretion of erythropoietin, which in turn increases red cell production
Anemia is a deficit in the mass of _____ ____, leading to reduced _______ delivery.
Anemia is measured by _______ ______ or _______
Oxygen saturation in anemia is ______ than normal, therefore the oxygen affinity ______ to help offset RBC mass deficiency
Anemia is a deficit in the mass of circulating RBCs, leading to reduced oxygen delivery.
Anemia is measured by hemoglobin concentration or hematocrit (RBC volume to total blood volume)
Oxygen saturation in anemia is lower than normal, therefore the oxygen affinity decreases to help offset RBC mass deficiency
Clinical Manifestations of Anemia
Mild to Moderate
Severe Anemia
Hemolytic Anemia
Mild to Moderate: often asymptomatic but can have breathlessness (dyspnea) and fatigue upon exercise
Severe Anemia: dyspnea and fatigue, pallor in nails, conjunctiva and buccal mucosa, tachycardia at rest
Hemolytic Anemia: splenomegaly because too many defective RBC’s accumulate in the spleen, Jaundice
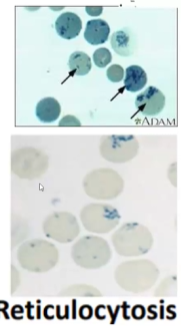
What test do you use to differentiate among anemia types such as hemolytic anemia, acute blood loss and decreased RBC production?
Reticulocyte Count (young RBCs)
If reticulocytes high → hemolytic or acute blood loss
If reticulocyte count low → decreased RBC production
Anemia due to Blood Loss in Acute vs Chronic
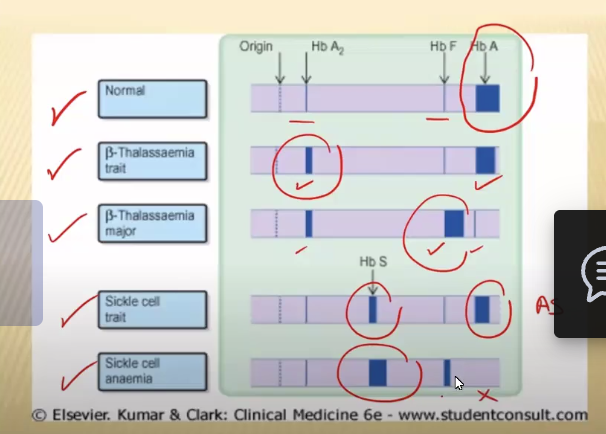
Acute: hematocrit and hemoglobin initially seem normal → THE ONLY normocytic monochromic anemia!!!
Chronic: gradual onset until iron deficiency is developed → microcytic hypochromic
Microcytic vs. Macrocytic vs. Normocytic
Microcytic: small red cell volume (MCV) and decreased mean red cell hemoglobin
Due to iron deficiency, thalassemia, Hb defect
Macrocytic: RBCs larger than normal
Due to folate or B12 deficiency, hemolytic, BM failure, liver disease, alcoholism, hypothyroidism
Can be Megaloblastic
Normocytic: RBCs are normal but there aren’t enough of them
Can be due to primary bone marrow problem such as aplasia or due to Bm replacement by leukemia, fibrosis or granulomas
Anemia due to Increased Red Cell Destruction (Hemolysis)
Two types
Examples
Extravascular hemolysis
Increased phagocytosis of RBCs in SPLEEN
Causes hyperbilirubinemia, jaundice and splenomegaly
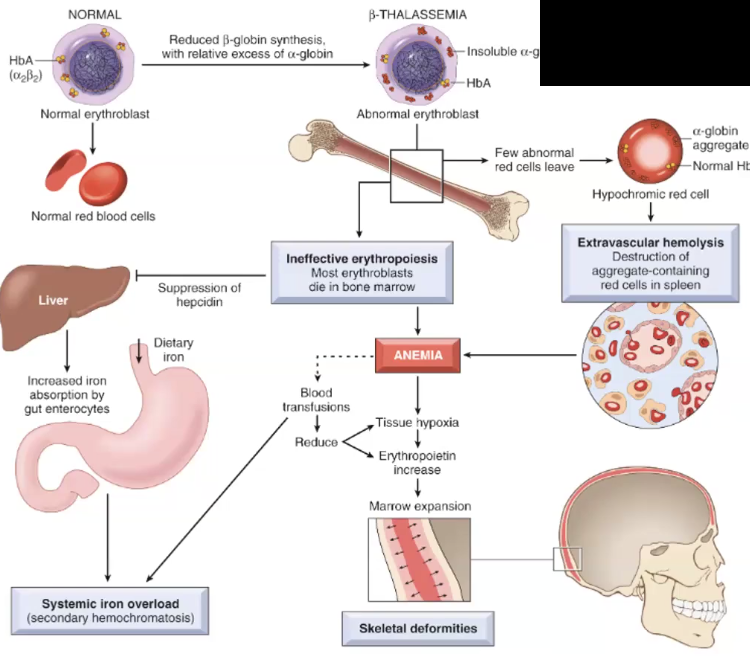
BETA THALASSEMIA IS EXTRAVASCULAR HEMOLYSIS!
Intravascular hemolysis
Increased RBC destruction due to mechanical forces like turbulent flow OR due to biochemical agents such as immune response
Erythropoiesis remains efficient
Hereditary Spherocytosis (HS)
What is it
Clinical Manifestations
Hemolysis is extravascular or intravascular?
Autosomal dominant mutation of membrane SPECTRIN proteins affecting vertical connections between membrane skeleton and band3 → causes cells to lose ability to bend, they become spherical and get stuck, then macrophages eat them
Moderate Anemia, Splenomegaly
EXTRAVASCULAR HEMOLYSIS
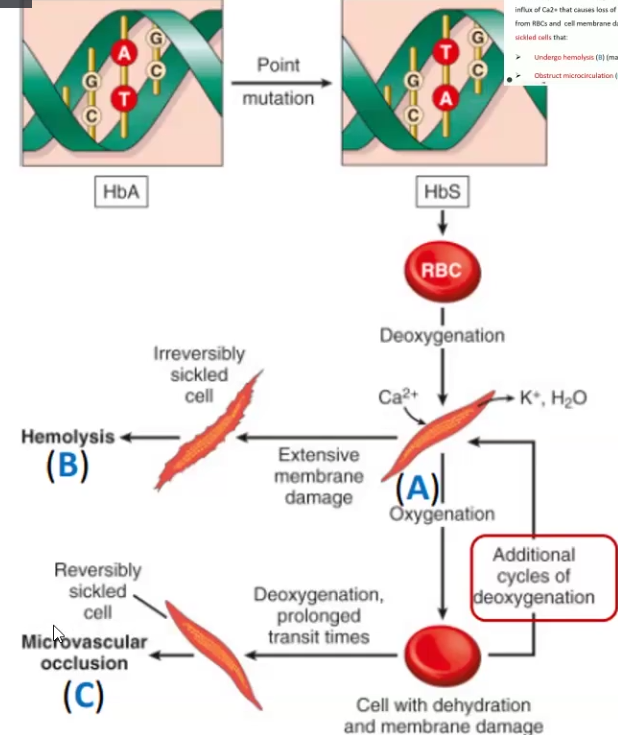
Sickle Cell Anemia
What is it
Clinical Manifestations
What test is required for definite Sickle Cell Anemia or Thalassemia diagnosis?
A hereditary disease caused by a point mutation is the beta-globin gene and results in abnormal Hb (HbS(sickle))
Homozygous is severely affected
Cells undergo hemolysis or obstruct microcirculation
Hemolytic anemia, jaundice, Ischemia
Gel Electrophoresis
Alpha Thalassemia
Causes
Clinical Manifestations
Caused by DELETIONS of one or more alpha globin genes → Severity depends on the number or genes
Can be a silent carrier with only 1 mutation; can be all 4 genes = Hydrops Fetalis; or can be Alpha Thalassemia Trait with 3 alpha globin gene deletions and one hemoglobin BUT HAVE NO SYMPTOMS
Beta Thalassemia
Cause
Clinical Manifestations
Cause
Beta Thalassemia MAJOR: very low or absent beta globin chains, causing the RBC to die in the bone marrow → ineffective erythropoiesis , which causes decreased hepcidin in the liver, leading to decreased intestinal iron absorption
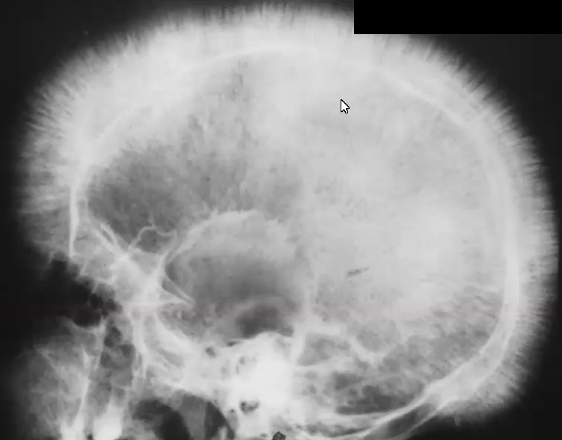
Clinical Manifestations
MAJOR: Hair-on-end appearance in skull, postnatal manifestation, growth retardation
INTERMEDIA: moderate severity, anemia
MINOR: asymptomatic
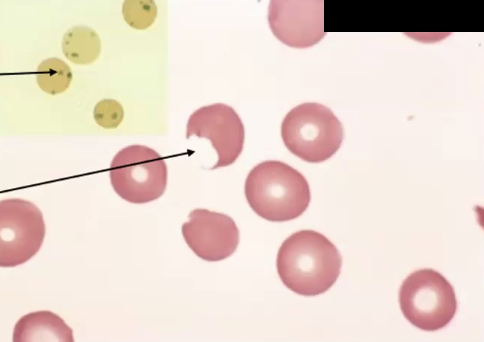
Glucose-6-Phosphate Dehydrogenase (G6PD) Deficiency
Causes
Clinical Manifestations
X-linked mutation where G6PD is deficient therefore NADPH (ANTIOXIDANT) production is affected and cells undergo oxidative stress→ intravascular hemolysis → can be due to infection, fava beans, drugs
Heinz bodies, Bite cells, intravascular hemolysis and extravascular hemolysis, anemia, splenomegaly, jaundice, hemoglobinemia, hemoglobinuria (
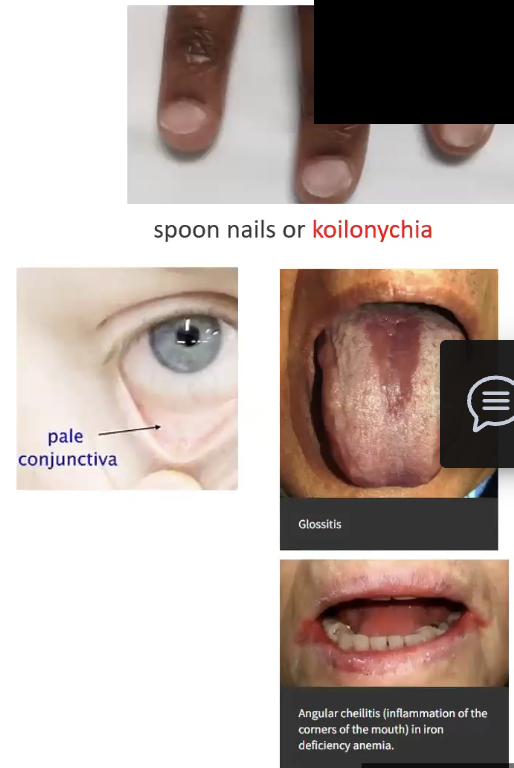
Iron Deficiency Anemia
Leads to what kind of anemia
Pathogenesis
What is an indicator that iron storages status?
Clinical Manifestations
Leads to hypochromic microcytic anemia
Pathogenesis: low iron levels cause inadequate hemoglobin production, erythroid activity increases, anemia appears once iron storage is completely depleted
Ferritin is proportional to amount of iron
Clinical Manifestations: Pallor, dyspnea, fatigue tachycardia, concave nail beds (spoon) (koilonychia)
Vitamin B12 Deficiency
Why is it important
Pathogenesis
What type of anemia does it cause
Clinical Manifestations
Vitamin B12 comes from animal products and is required for synthesis of FH4, which is crucial for DNA synthesis
Can be due to
Autoimmune attack on gastric mucosa → Pernicious anemia
Anti-parietal cell antibodies
Malabsorption
MEGALOBLASTIC ANEMIA
Anemia, Neurological symptoms such as demyelination of the spinal chord, malabsorption, low WBC and platelets, Pallor
Folate Deficiency
Why is it important
Pathogenesis
What type of anemia does it cause
Clinical Manifestations
Folate is present in. fruits, vegetables, leafy greens,liver and meat, folate depletes quick because it has a small reserve, it is essential for DNA synthesis
Pathogenesis: Suppressed DNA synthesis leads to decreased reticulocytes
Megaloblastic Anemia
Anemia, low WBC, low platelets, malabsorption, increased risk of neural defects during pregancy, NO NEUROLOGICAL SYMPTOMS!
Polycythemia
What is it
Causes
Types
Abnormally high number of circulating RBCs
Types
Relative: due to hemoconcentration bc of loss of plasma volume → dehydration, vomiting, diarrhea, diuretic
Absolute: Increased total red cell mass
Primary: due to intrinsic abnormality of hematopoietic precursors, uncontrolled RBC proliferation independent of erythropoietin levels → often polycythemia vera aka myeloproliferative neoplasm
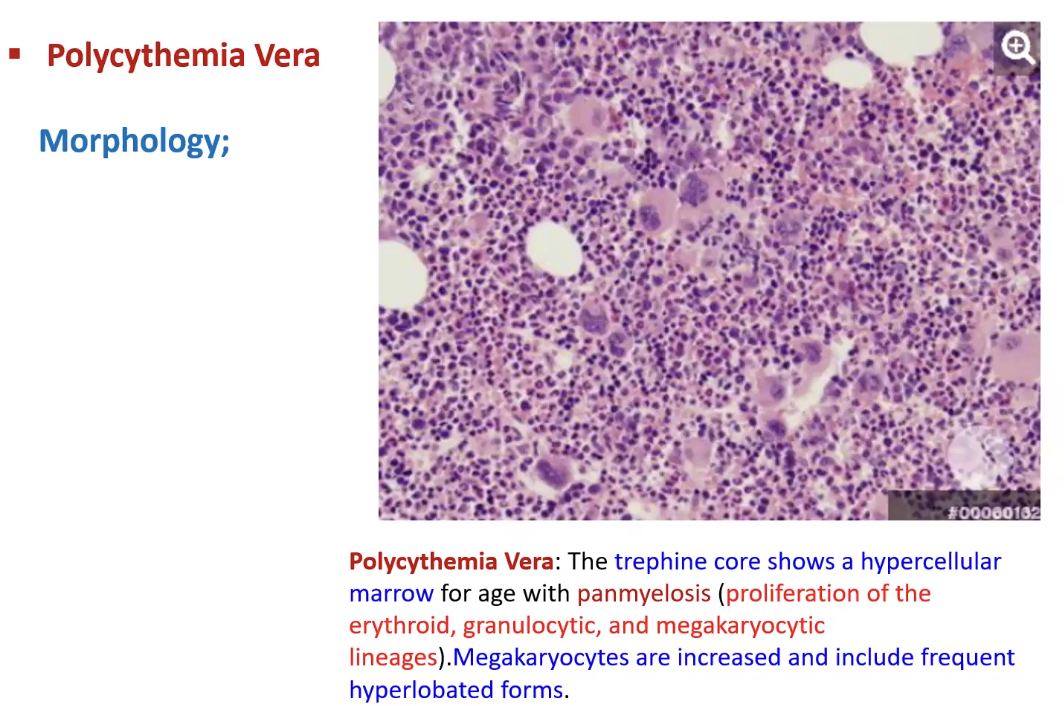
Secondary: due to elevated erythropoietin levels → high altitude, smoking, lung disease, cyanotic heart disease
Non-Neoplastic Disorders of WBCs
Leukopenia: low WBC count
Neutropenia: low neutrophils, causes agranulocytosis
Lymphopenia
Leukocytosis: elevated WBC count
Neutrophilic leukocytosis
Eosinophilic leukocytosis
Basophilic leukocytosis
Monocytosis
Lymphocytosis
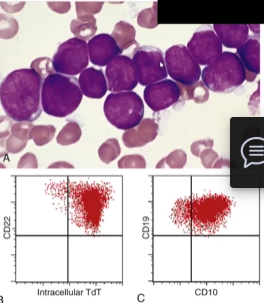
Acute Lymphoblastic Lymphoma/Leukemia (ALL)
What is it
Causes
Clinical Manifestations
Markers for T vs B cells
Neoplasms that cause uncontrolled proliferation of immature B and T cells (lymphocytes) → most are B cells (B-ALL) → most common cancer in children
Caused by gene aberrations in transcription factors for B and T cell development
Clinical Manifestations:
Hypercellular Bone Marrow packed with more than 20% lymphoblasts that have condensed chromatin, small nucleoli and are agranular
Fatigue, fever, Bleeding, Testicular enlargement, CNS manifestations, headache, vomiting, nerve palsies
B cells have CD22, CD10 and CD19+ markers, while T cells have CD1, CD2, CD5 and CD7
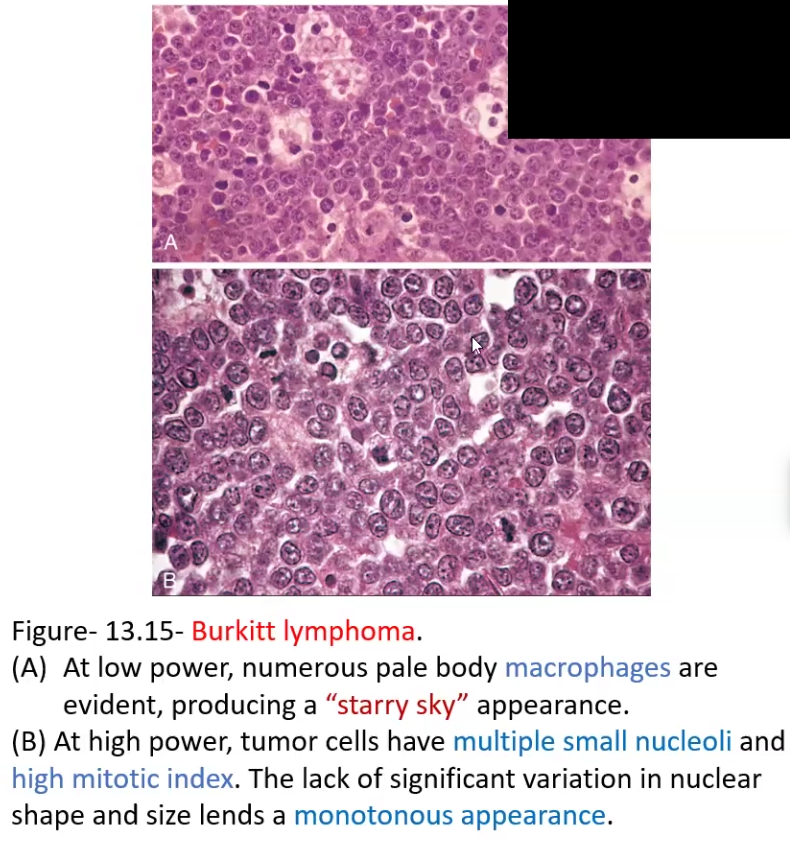
Burkitt Lymphoma
What is it
Causes
Types
Histologic Patterns
Clinical Manifestations
Peripheral B Cell neoplasm that derives from mature peripheral cells with different degrees of maturation
Cause: gene translocation activates oncogene and creates Philadelphia chromosome, increasing MYC protein levels
Can be associated with EBV
Types: Sporadic, Endemic, Aggressive Lymphoma in individuals with HIV
Histologic Patterns: Starry Sky
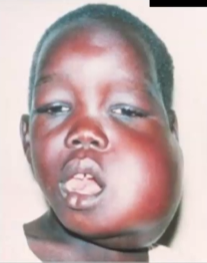
Clinical Manifestations: Tumors in extra nodal sites → mandibular tumor or mass in ileocecum and peritoneum
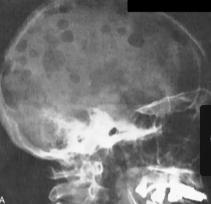
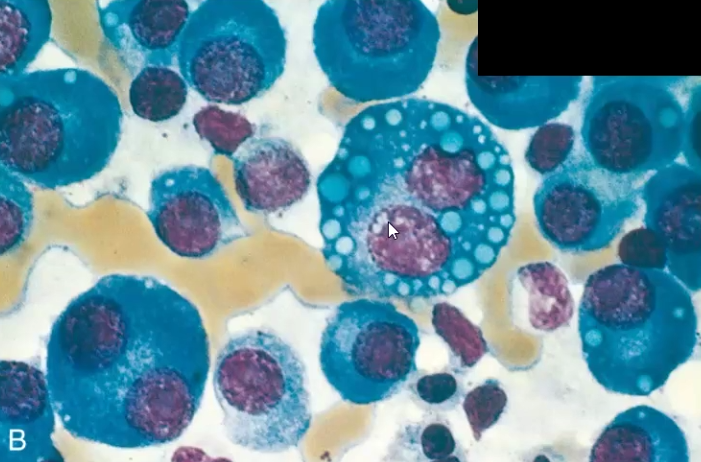
Multiple Myeloma
What is it
Clinical Manifestations
Plasma cell abnormality due to chromosomal abnormalities that increase their proliferation
Increased IL-6, IL-3 induces osteoclastic activity and increased DKK-1 suppresses osteoblast activity → leads to lytic bone lesions
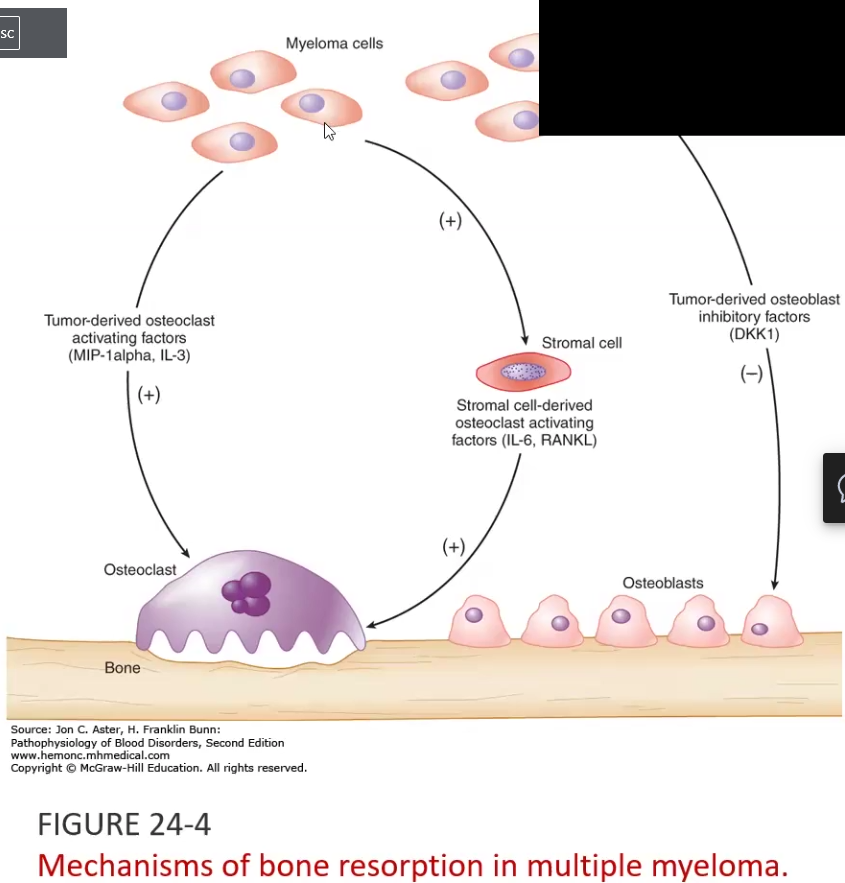
Clinical Manifestations: Multifocal destructive skeletal lesions such as in the vertebral column, ribs, skull, pelvis, femur, clavicle and scapula
Normocytic monochromic anemia
Renal failure: Bence Jones proteins in urine
Hodgkin Lymphoma
What is it
Clinical manifestation
Neoplasm of the blood characterized by Reed-Sternberg (RS) cells → giant cells with mirror acidophilic nuclei and slightly basophilic cytoplasm
Usually found in a single lymph node, then it spreads
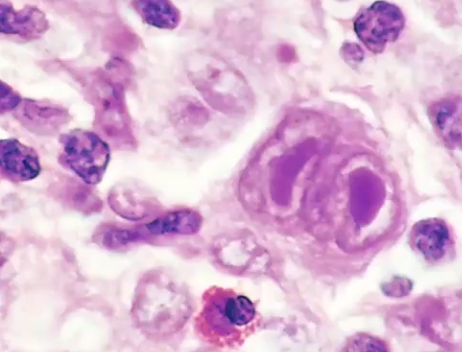
Painless lymphadenopathy, fever, night sweats, weight loss
Acute Myeloid Leukemia (AML)
What is it
Histologic Pattern
Clinical manifestations
A Myeloproliferative neoplasm associated with radiation, chemotherapy and benzene exposure, cigarette smoking → drive by mutations that block myeloid cell differentiation (monoblasts, erythroblasts, megakaryocytes)
Auer rods (need-like structures)
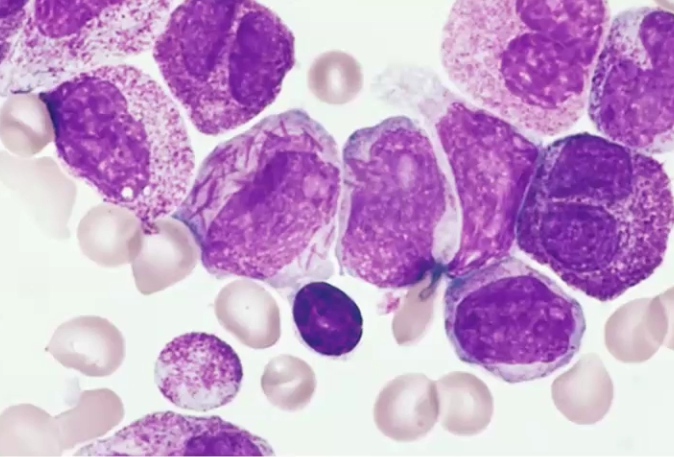
Anemia, thrombocytopenia, neutropenia, gingival hyperplasia
Chronic Myeloid Leukemia (CML)
What is it
Clinical manifestations
Phases
Myeloproliferative disorder (mainly granulocytes) caused by the translocation and fusion of BCR-ABL leading to Philadelphia chromosome → leads to constitutive activity of tyrosine kinase that mimics growth factors
Hypercellular bone marrow
Phases
Chronic Phase → 3-5 years no symptoms other than elevated WBC
Accelerated Phase → fever, night sweats, splenomegaly, bone pain, chromosomal abnormalities
Blast crisis Phase → resembles AML
Define
Ischemia
Infarction
Aneurysm
Ischemic Heart Disease (IHD)
Angina Pectoris
Myocardial Infarction
Ischemia: decrease in blood supply to tissues resulting in restriction and reduction of the availability o nutrients and the removal of metabolic wastes
Infarction: area of ischemic necrosis within a tissue or organ produced by occlusion of either arterial supply or venous drainage
Aneurysm: a localized abnormal dilation of a blood vessel of the heart
Ischemic Heart Disease (IHD): group of syndromes caused by myocardial ischemia
Angina Pectoris: one of the clinical syndromes of IHD, means chest pain where ischemia is not severe enough to cause infarction
Myocardial Infarction: IHD clinical syndrome caused by ischemic necrosis of the myocardium
3 Types of arteries
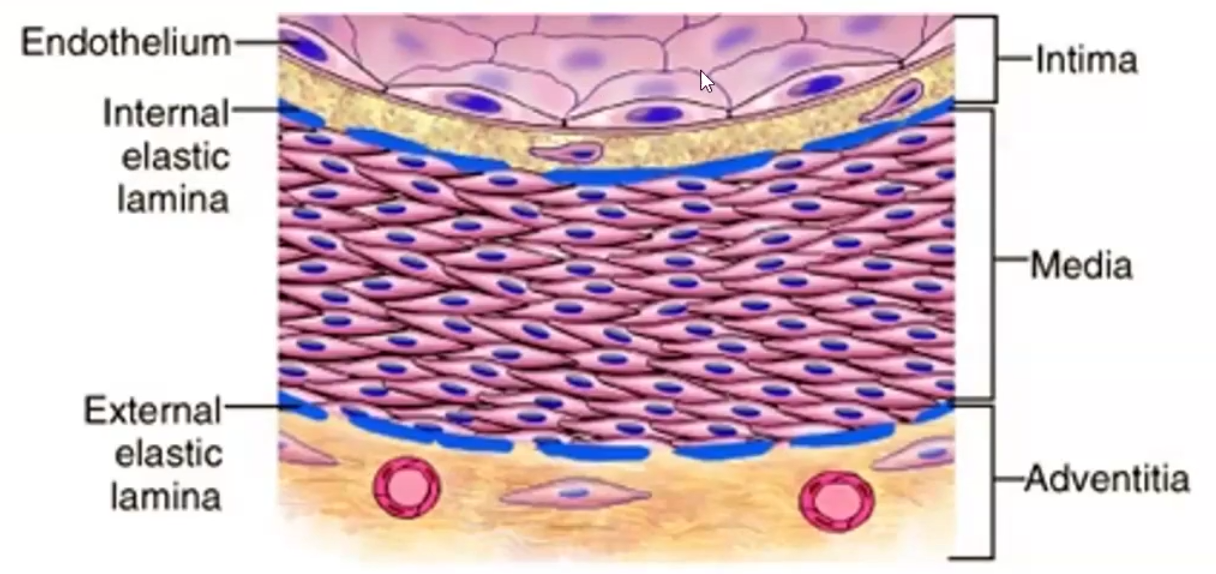
Constituents of the walls of blood vessels, layers
Large elastic, Medium muscular, small
Made of endothelium (intima), smooth muscle cells (media) and extracellular matrix (adventitia)
Atherosclerosis
Mockberg’s medial calcific sclerosis
Arteriolosclerosis
Atherosclerosis: hardening of arteries
Mockberg’s medial calcific sclerosis; calcific deposits
Arteriolosclerosis: hardening of arterioles
Hyaline
Hyperplastic: smooth muscle hyperplasia due to malignant hypertension → onion skin appearance
Atherosclerosis
What is it characterized by
Causes harm by
Characterized by intimal lesions called atheromas, atheromatous or fibrofatty plaques which protrude and obstruct vascular lumens and weaken underlying media
Causes harm by
Chronically decreasing blood supply
Occluding arteries suddenly by rupture of plaques
Weakening of walls tunica media causing atherosclerotic aneurysms
Atherosclerosis Non-Modifiable vs Controllable Factors
Non-modifiable
Increasing age
Male sex
Family history
Genetic abnormalities
Controllable
Hyperlipidemia
Hypertension
Smoking
Diabetes Mellitus
Inflammation
Hypercholesterolemia particularly due to ____ cholesterol is a major risk factor for Hyperlipidemia
______ cholesterol, in contrast, lowers the risk by mobilizing cholesterol fro, atheroma to the liver and excreting it through the bile
Hypercholesterolemia particularly due to LDL cholesterol is a major risk factor for Hyperlipidemia
HDL cholesterol, in contrast, lowers the risk by mobilizing cholesterol fro, atheroma to the liver and excreting it through the bile
Atherosclerosis Pathogenesis
Endothelial injury → endothelial dysfunction
Macrophage activation, migration of tunica media to intima
Fat is engulfed
Results in fatty core and fibrous cap
Thin cap plaques are the most prone to plaque _____, generally leading to sudden _______ _____.
Stable plaques can undergo surface _____ and _____, rapidly expanding the plaque size and leading to prominent _______. This event can lead to sudden ______ _____.
Extensive narrowing of the luminal diameter drom large plaques generally results in ______ _____, reducing blood supply to the heart and resulting in _____.
Atherosclerotic plaque develop primarily in
The key process in atherosclerosis is ______ thickening and _____ accumulation
Thin cap plaques are the most prone to plaque rupture, generally leading to sudden cardiac death.
Stable plaques can undergo surface erosion and thrombosis, rapidly expanding the plaque size and leading to prominent calcifications. This event can lead to sudden cardiac death.
Extensive narrowing of the luminal diameter from large plaques generally results in critical stenosis, reducing blood supply to the heart and resulting in angina.
Atherosclerotic plaque develop primarily in elastic arteries and large/medium sized muscular arteries
The key process in atherosclerosis is intimal thickening and lipid accumulation
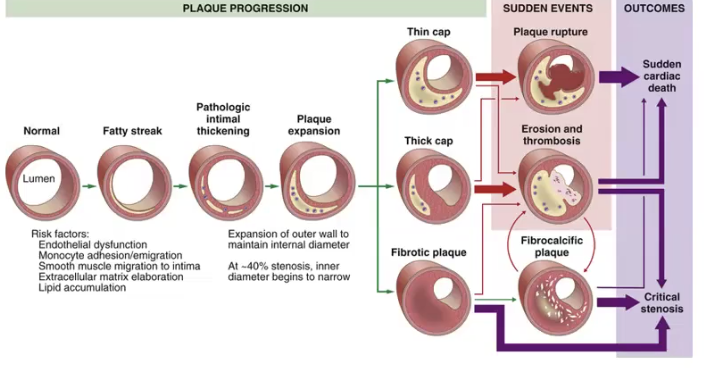
The fibrous cap is composed of
The necrotic core consists of
The fibrous cap is composed of macrophages, smooth muscle cells, lymphocytes
The necrotic core consists of cellular debris, extracellular lipid with cholesterol crystals and foamy macrophages
Ischemia not only limits tissue __________, but also reduces availability of _______ and the removal of ________ _______
In more than 90% of cases, myocardial ischemia results from ______ _____ _____ due to obstructive atherosclerotic lesions in the epicardial coronary arteries
In addition to coronary atherosclerosis, myocardial ischemia can be caused by:
Ischemia not only limits tissue oxygenation, but also reduces availability of nutrients and the removal of metabolic wastes
In more than 90% of cases, myocardial ischemia results from reduced blood flow due to obstructive atherosclerotic lesions in the epicardial coronary arteries
In addition to coronary atherosclerosis, myocardial ischemia can be caused by: coronary emboli, myocardial vessel inflammation and vascular spasm
Angina Pectoris
Define
What is the pain due to
3 Types
Angina Pectoris: characterized by paroxymal and usually recurrent attacks of substernal or sternal discomfort caused by transient (15 s to 15min) myocardial ischemia that is insufficient to induce myocyte necrosis
Pain is likely a consequence of adenosine, bradykinin and other sympathetic and vagal stimulus
3 Types:
Stable/Typical: usually caused by stress, excitement, anxiety, and is due to a perfusion imbalance, improves with rest → EKG shows transient ST segment depression bc ischemia is subendocardial
Unstable or crescendo: increasingly frequent or severe caused by plaque → EKG shows depressed ST segment
Prinzmetal variant angina: episodic myocardial ischemia caused at rest by coronary artery spasm → EKG shows elevated ST segment (transmural ischemia)
What are some Causes of Myocardial Infarction?
Coronary Artery Occlusion
Vasospasm
Emboli
Due to the myocardial perfusion pattern from epicardium to endocardium, ischemia is most pronounced in the ____________, thus, irreversible injury of ischemic myocytes occurs first in the ___________ zone
The MI is transmural when ___ than 50% of the myocardial wall is affected by the ischemic necrosis → shows ________ ST segment
The Mi is subendocardial when ___ than 50% of the myocardial wall is affected by the ischemic necrosis → Shows _______ ST segment
Due to the myocardial perfusion pattern from epicardium to endocardium, ischemia is most pronounced in the subendocardium, thus, irreversible injury of ischemic myocytes occurs first in thevsubendocardial zone
The MI is transmural when more than 50% of the myocardial wall is affected by the ischemic necrosis → shows elevated ST segment
The Mi is subendocardial when less than 50% of the myocardial wall is affected by the ischemic necrosis → Shows depressed ST segment
What are specific biomarkers used to detect myocardial damage? What is the timeline of when they are present
All present between 3-12 hours after event and continue to be elevated 7-10 days after
Cardiac specific troponins T and I → cTnT and cTnI
MB fraction of creatine kinase → CK-MB
The most common congenital valve disorder is related to the _____ _______ valve, but it usually doe not result in _____ or ________ in early life, instead it is more prone to progressive degenerative _________ that gives rise to ______
________ stenosis of the aortic and mitral valves account for approximately 2/3rd of all valve disease
The most common congenital valve disorder is related to the bicuspid aortic valve, but it usually doe not result in stenosis or incompetency in early life, instead it is more prone to progressive degenerative calcification that gives rise to stenosis
ACQUIRED stenosis of the aortic and mitral valves account for approximately 2/3rd of all valve disease
Two Types of Degenerative Valve Disease
Calcification/Fibrosis: cuspal or annular masses that obstruct valvular opening leading to stenosis
Inevitable with age
Most common cause of aortic stenosis
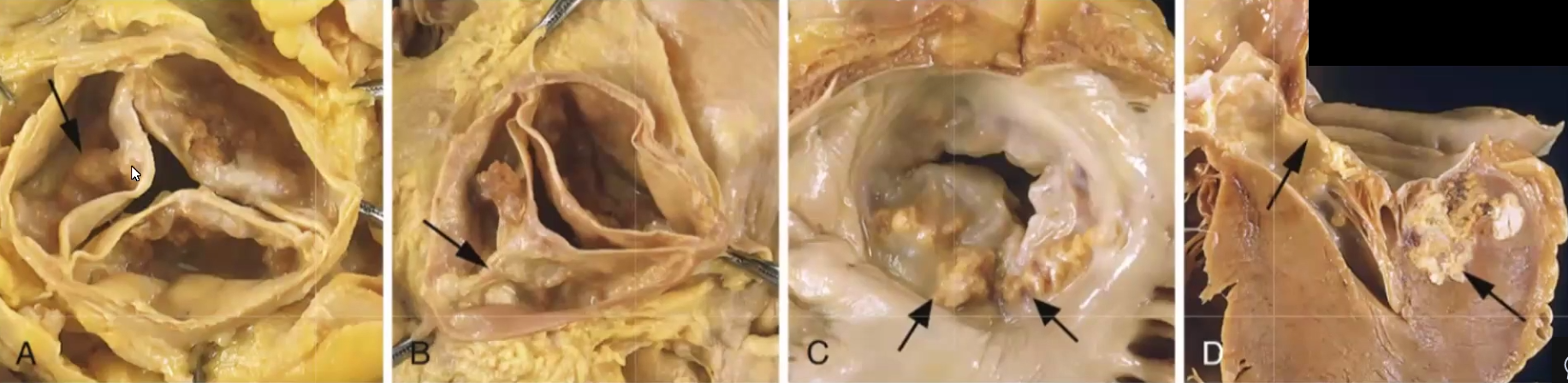
Myxomatous: causes floppy valves that balloon back into left atrium
Uncommonly associated to Marfan syndrome (fibrilin1 mutation)
Midsystolic click and regurgitant murmur
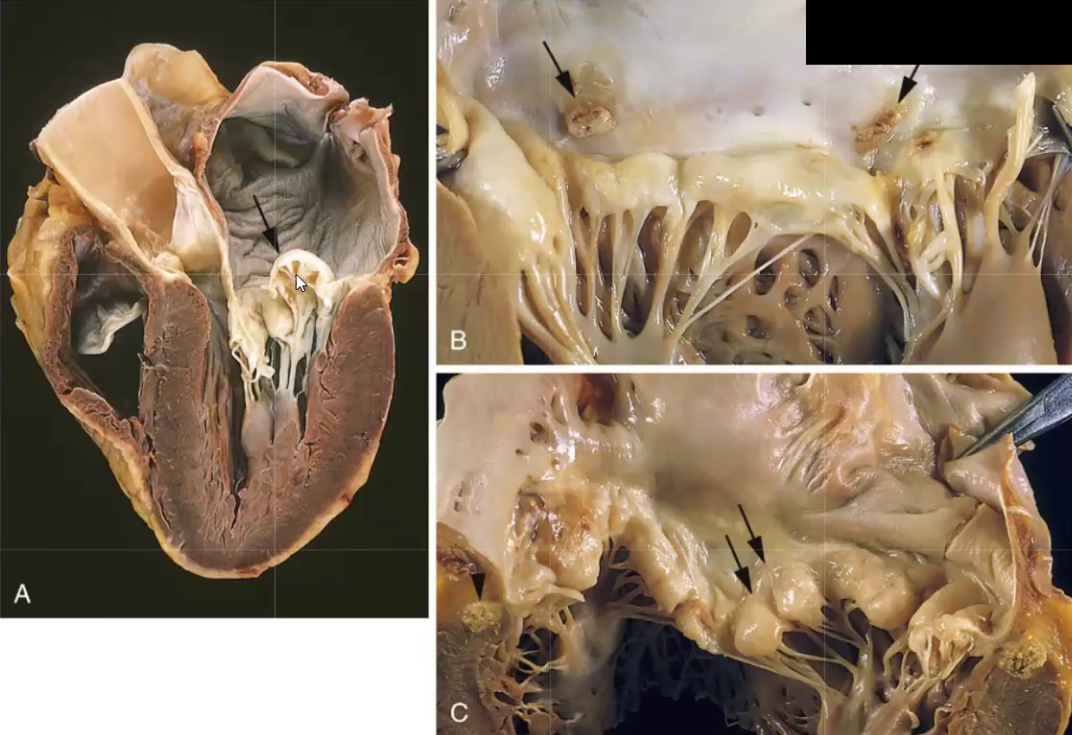
Rheumatic Fever
What causes it
What can it progress to
What cells and bodies are found in heart tissue affected by rheumatic fever?
Commonly preceded by streptococcal group A pharyngitis, then CD4+ T cells attack Strep M proteins, but molecular mimicry causes T cells to confuse myocardial cells with M proteins and an autoimmune reaction occurs destroying the heart → results in pancarditis (all layers inflammed)
Can progress to acute rheumatic carditis, where mitral valve will deform and can progress further to Rheumatic Heart Disease (leaflet thickening, fusion of tendinous chords, “fishmouth” stenosis)
Aschoff bodies and Anitschkow cells:
Infective Endocarditis
What is it
Types
A microbial infection (usually extracellular bacteria) Types of the heart valves or mural endocardium that leads to large vegetations and destruction of underlying tissue
Types
Acute endocarditis: tumultuous destructive infections attacking previously normal valve
10-20% caused by Strep. aureus, some because of IV drug use
Subacute endocarditis: slow, low virulence infections affecting previously abnormal heart valves
AKA pre-existing defect makes you prone to bacterial endocarditis
50-60% caused by Strep Viridans
Among the factors predisposing to endocarditis is seeding of the ______ with ________.
The mechanism or portal of entry of the agent into the bloodstream may be an obvious _______ elsewhere, a ________ procedure that causes transient bacteremia, injection of contaminated bacteria directly to the bloodstream by _______ ___ ________.
The most susceptible are the ____ and ____ valves although the ________ valve can be a frequent target as well for IV drug users
Among the factors predisposing to endocarditis is seeding of the blood with microbes.
The mechanism or portal of entry of the agent into the bloodstream may be an obvious infection elsewhere, a dental procedure that causes transient bacteremia, injection of contaminated bacteria directly to the bloodstream by intravenous drug use.
The most susceptible are the aortic and mitral valves although the tricuspid valve can be a frequent target as well for IV drug users
Malignant Hypertension
What is it
Parameters
What does it cause
It is a rapidly rising and severe hypertension that can happen in normotensive persons but more often is superimposed on preexisting benign hypertensions
Systolic pressure >180mmHg and diastolic pressure >120mmHg
Renal failure, retinal hemorrhages and exudates
Essential Hypertension
What is it
Mechanisms/possible causes
Consequences
it is a hypertension that is idiopathic, that is it occurs by itself, not a consequence of another disease
Genetic factors, environmental factors, reduced sodium excretion, vasoconstrictive influences
Hyaline arteriosclerosis, hyperplastic arteriolosclerosis → but if left untreated it can have the same symptoms as malignant
Hyaline vs Hyperplastic arteriolosclerosis
First damage seen in vasculature as a consequence of hypertension
Hyaline: homogenous pink hyaline thickening, luminar narrowing → also seen in diabetics
Hyperplastic: only in severe hypertension, the vessels become laminated with fibrinoid deposits/necrosis like an onion and the lumen narrows, mostly occurs in the kidney
Left-Sided Heart Failure
What is it
Most common causes
Consequences
Heart failure that can affect left side but eventually can lead to right side failure as well
The most common causes are ischemic heart disease, systemic hypertension, mitral or aortic valve disease, primary myocardial disease
The consequences are diminished systemic perfusion and elevated pulmonary circulation pressure → pulmonary congestion and edema produce heavy wet lungs, perivascular edema, edematous alveolar septa, accumulation of edema in alveolar space, cardiomegaly, NOT LOWER BODY EDEMA
Right-Sided Heart Failure and Cor Pulmonale
Most common causes
Consequences of Cor Pulmonale
Most times is a consequence of left side failure but when isolated (Cor Pulmonale), it is due to disorders affecting the lungs
Consequences include engorgement of systemic and portal venous systems, no pulmonary congestion, right ventricular hypertrophy/dilation, pulmonary embolism, lung disease of parenchyma
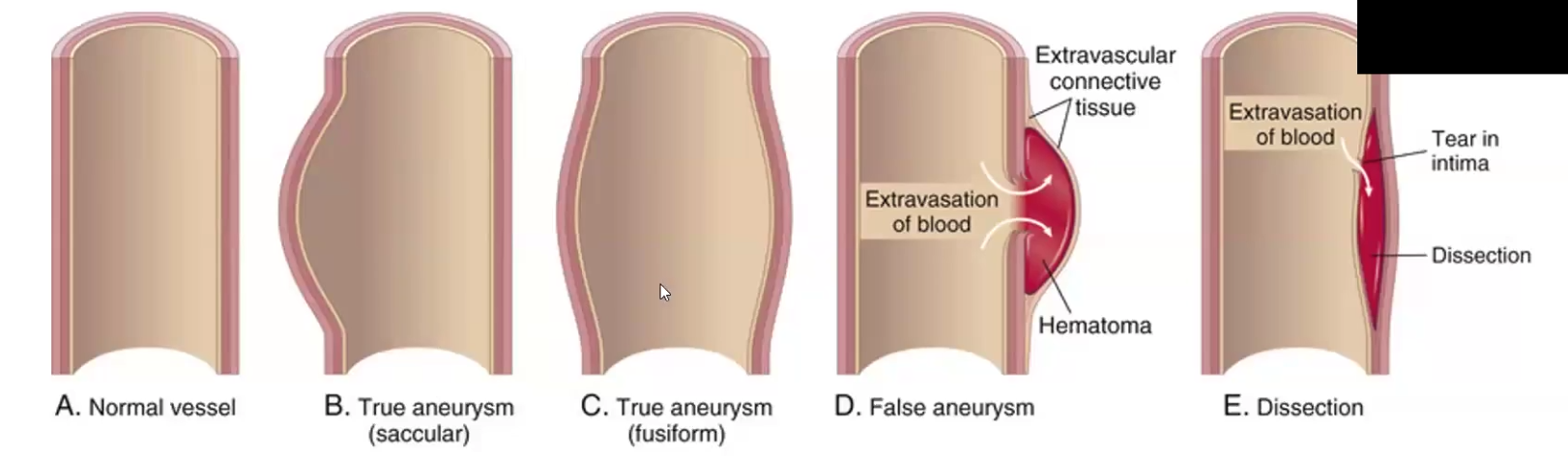
Aneurysm
Define
True vs. False aneurysm
Due to what
Aneurysm is a localized abnormal dilation of a blood vessel or the heart that may be congenital or acquire → weakening of underlying media
True: the vascular wall is attenuated/weak, and it bulges out
False: extravascular hematoma that freely communicates with intra-vascular space
Can be due to CT abnormality like Marfan’s Syndrome or can be due to hypertension and atherosclerosis
Arterial dissection
Define
Causes
Morphology
Symptoms
When blood enters a defect in the arterial wall and tunnels between layers
Can be due to CT abnormality like Marfan’s Syndrome or can be due to hypertension and atherosclerosis → HYPERTENSION IS THE MAJOR RISK FACTOR!
Double barrel appearance, absent inflammation
Sudden excruciating pain on chest, radiating to the back and moving down
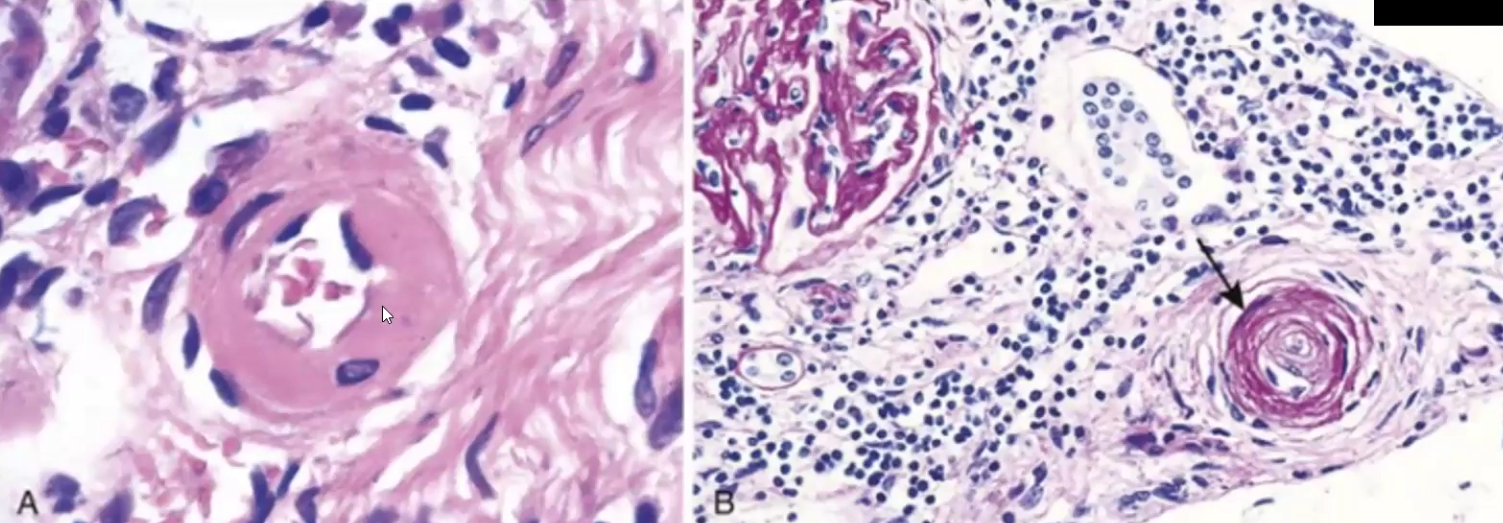
Takayasu Arteritis
What is it
Symptoms
Autoimmune granulomatous inflammation of the aorta occurring in young Asian females
Weak pulse, visual defects
Kawasaki Disease
What is it
It is an infectious triggered immune reaction that is delayed hypersensitivity that causes self-limited acute febrile disease in children, usually of Japanese descent
Pyogenic Granulomas
Capillary hemangiomas present as rapidly growing red pedunculated lesions on the skin, gingival and oral mucosa
Pregnant women
Pathological Responses of the Glomerulus to Injury
Hypercellularity: proliferation of mesangial and endothelial cells and formation of crescents
Basement Membrane Thickening: deposition of dense material and increased synthesis of protein
Hyalinosis and Sclerosis: accumulation of hialine cartilage and deposition of extracellular collagenous matrix
Primary vs. Secondary Glomerular disease
Primary: disorders in which the kidney is the only or predominant organ involved
Primary: causes by another disease such as SLE
Nephrotic vs Nephritic Syndrome
Nephrotic: proteinuria >3.5, hypoalbuminemia <3, hyperlipidemia, edema
Nephritic: hematuria, mild proteinuria <3.5, renal failure, hypertension, smokey urine
Acute Proliferative Glomerulonephritis
Immune complex containing streptococcal antigens form and deposit in BM
Usually presents as nephritic syndrome with smoky urine/hematuria after strep throat
Rapidly Progressing Glomerulonephritis
Rapid and progressive loss of renal function, severe oliguria (low urine output), poor prognosis
Pathogenesis includes anti-GBM (glomerular basement membrane) antibodies with linear deposits of IgG and C3
Can cause post-infection glomerulonephritis
Goodpasture Syndrome (Nephritic)
Caused by anti-BM antibodies
Clinical lung involvement including hemorrhage and hemoptysis precedes renal problems
Membranous Nephropathy
Characterized by diffuse thickening of the glomerular capillary wall due to accumulation of IgG
Chronic immune-complex mediated disease
Minimal Change Disease
Effacement of podocytes only seen on EM
Lipoid nephrosis or nil disease, most common nephrotic syndrome in children
Focal Segmental Glomerulosclerosis
Epithelial damage of glomerulus characterized by hyalinosis and sclerosis
Berger Disease (IgA Nephropathy)
IgA nephropathy caused by defective IgA (poorly glycosylated) and autoantibodies against this poorly formed IgA
Mainly nephritic but can be nephrotic
Lesions seen in Diabetic Nephropathy
Widespread thickening of the basement membrane
Renal and vascular arteriolosclerosis
Pyelonephritis (infection)
Urinary Tract Obstruction (Uropathy)
also define hydronephrosis
Lesions of the urinary tract cause obstruction and increase susceptibility to infection and stone formation
Hydronephrosis is the dilation of renal pelvis and calyces due to obstruction
In bilateral partial obstruction, what are the earliest manifestations?
What about complete obstruction?
Bilateral partial obstruction has inability to concentrate urine → Tubulointerstitial disorder
Oliguria and anuria → incompatible with life
Urolithiasis (Renal Calculi)
Form due to what
Stones show on radiograph as…
hypercalcemia and hypercalciuria, uric acid, cystine stones
Radiopaque
Autosomal Dominant Polycystic Kidney Disease
Mutations in PKD1 and 2 gene cause cysts that ultimately destroy both kidneys
One mutation is inherited and the other one is acquired in the somatic cells of the kidney
Small radially arranged cysts