10 - Regenerative medicine & gene therapy
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
31 Terms
Which of the following are true about CRISPR-based gene editing technology?
A) It is based on a bacterial immune defense
B) It requires an RNA guide sequence and an endonuclease protein
C) It results in cleavage of Cas9
D) It can be used for cleavage of a DNA target sequence based on complementary base pairing
E) It can only be used to edit bacterial DNA
F) Cas9 is a DNA repair enzyme that inserts a particular change in the genome
a,b,d
how can CRISPR/Cas9 be used for genome editing
CRISPR/Cas9 uses a guide RNA (gRNA) to direct the Cas9 nuclease to a specific DNA sequence, where it induces a double-strand break (DSB). The cell repairs this DSB in one of two main ways
genome editing with homology-directed repair
High-fidelity, but only active in certain cell cycle phases.
Requires a DNA template with homology to the break site.
Used to precisely edit DNA (e.g., fix point mutations, insert specific sequences).
genome editing with Non-homologous end joining (NHEJ)
Error-prone, quick.
Often results in insertions or deletions (indels).
Used to knock out genes (e.g., to stop expression).
Good for disrupting gene function.
Gene therapies
use introduction/alteration of DNA sequences to create a therapeutic benefit
Gene therapies are not new, but CRISPR/Cas9 is making this much easier and more precise, which is opening up new possibilities
Gene therapies using viruses
introduce new DNA sequences to at random sites in the genome have been around for awhile and are still used routinely
How is gene therapy using viruses different than CRISPR/Cas9?
gene therapy injects seq to bind to random sites, whereas CRISPR targets particular locations (not random)
Should it be legal to make changes in the human genome? If so, when/why? What purposes/goals are acceptable? What ethical issues can you imagine?
can be controversial
usually ethical issues when it can affect the germline
accessibility (finances: wealthier people would have more accessibility to edit their germline)
when used for vanity rather than genetic diseases
Regenerative medicine
seeks to restore tissue structure and function and provide options for what were previously untreatable injuries or diseases.
Would Regenerative medicine involve altering the germline DNA?
no
Regenerative medicine approaches can involve
cell therapy (transfer of live cells, often after modification/growth outside the body), gene therapy (gene editing), and/or tissue bioengineering.
What features would make a particular disease/condition a good target for development of CRISPR/Cas9 gene editing-based therapeutic approaches
Involve single-gene mutations (e.g., sickle cell disease).
disease negatively impacts quality of life and/ or lifespan
Have well-characterized genetic causes.
the cells causing the disease are easy to get rid of and replace (active and accessible stem cells)
why was 2023 a big year for crispr
treatment for sickle cell disease became the first CRISPR/Cas9 gene editing therapy to be FDA-approved
Sickle cell disease
an inherited blood disorder caused by a single nucleotide change that alters a single amino acid in the gene for β-globin, a subunit in hemoglobin. This causes abnormal clumped protein fibers.
Gene editing technology can allow sickle patients to be their own stem cell donor to continuously produce their own normal RBCs
how is crispr used in Sickle cell disease
The patient’s hematopoietic stem cells are isolated and edited to correct the single nucleotide change. These can then be re-introduced to the patient after chemotherapy to remove existing unedited hematopoietic stem cells.
What makes sickle cell disease a good candidate to cure through gene therapy?
caused by a single point mutation in the β-globin gene.
Affects a well-defined, accessible cell type (hematopoietic stem cells).
Edited stem cells can be autologously reintroduced, reducing rejection.
FDA approved therapy (Casgevy, 2023) shows safety and efficacy.
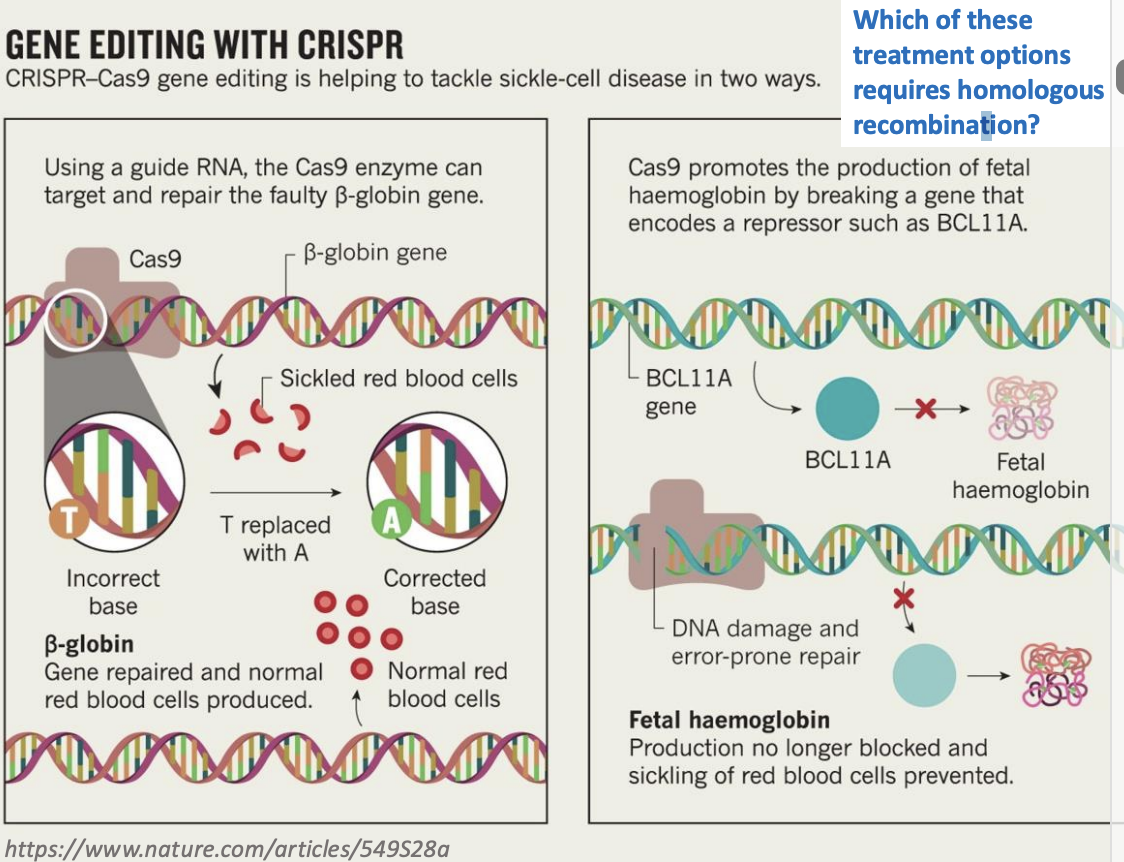
Which of these treatment options requires homologous recombination?
option a, since it usesd a guide rna to repair the faulty b-globin gene
Are all stem cells the same?
no
Yamanaka factors
Oct4, Sox2, Klf4, c-Myc.
These reprogram somatic cells into Induced pluripotent stem cells (iPSCs), which are pluripotent.
They are master regulators of pluripotency!
Which of the research techniques discussed earlier in the course would help with a study involving Yamanaka factors
rna-seq
Why was the discovery of iPSCs so important?
it can be used for anyone
you dont need to harvest from and potentilaly kill an embryo to get cell
How else might the ability of master regulators to redirect cell fate be useful in human health?
Since master regulators can be utilized to reprogram cells into pluripotent stem cells, they can then be directed to differentiate into specific cell types needed for regenerative therapies, potentially treating a variety of diseases and injuries.
source of hematopoietic stem cells
Cord blood is FDA approved as a source of hematopoietic stem cells to treat diseases of the blood/immune system (ex. leukemia and sickle cell anemia)
Which of the following are TRUE about stem cell therapies and gene editing?
A) Umbilical cord blood is an FDA-approved source of stem cells for treating spinal cord injury
B) A somatic cell could be reprogrammed to a pluripotent state by expression of the Yamanaka factors
C) The CRISPR gene editing sickle cell disease therapy involves making a genetic change in the patient’s own hematopoietic stem cells and then reintroducing those cells back into the patient
D) Regenerative medicine approaches modifying tissues/somatic cells are unlikely to be passed on to offspring
E) Altering genes using CRISPR/Cas9 and homology-directed repair can result in errors and off-target effects
F) Most people find it ethically preferable to edit embryos/germline cells instead of somatic cells
b,c,d,e
Distinguish between germline and somatic cell editing
Germline editing: Alters DNA in eggs/sperm → inheritable changes.
Somatic editing: Alters DNA in body (non-reproductive) cells → non-heritable.
germline and somatic cell editing: ethical and health-related issues
Germline editing:
High ethical concerns: unpredictable long-term effects, societal implications.
Currently banned in many countries.
Somatic editing:
More accepted.
Used in approved therapies (e.g., sickle cell disease treatment).
Cell therapy
Transplanting cells (often modified or grown in vitro) to treat disease.
Stem cell sources:
Embryonic stem cells (ESCs): pluripotent.
Induced pluripotent stem cells (iPSCs): reprogrammed somatic cells.
Adult stem cells (e.g., hematopoietic from bone marrow or cord blood).
why therapeutic efficacy differs for different types of stem cells and different diseases/tissues
Different stem cells have different differentiation potentials.
Some tissues regenerate well (e.g., blood); others do not (e.g., brain).
iPSCs might not function identically to ESCs.
Tissue environment, immune response, and gene expression compatibility also matter.
Apply concepts surrounding commitment and differentiation to explain why all cells are not equally useful for all potential applications in regenerative medicine
Differentiated cells are committed and cannot change fate.
Pluripotent stem cells can become any cell type—ideal for regeneration.
The more committed a cell is, the fewer options for therapy.
Example: muscle cells can’t become neurons without full reprogramming.
Apply concepts in gene regulation/mutation to identify ways that gene therapy approaches could be helpful in treating or studying human diseases.
Loss-of-function mutations can be treated by inserting a normal copy of the gene (e.g., using viral vectors).
Gain-of-function or dominant-negative mutations might benefit from CRISPR/Cas9 knockout or correction.
CRISPR can be used in research to:
Study gene function by knockout/knock-in.
Model human diseases in cells or animals.
Screen for drug targets via genome-wide CRISPR libraries.