Somitogenesis: Development of Paraxial Mesoderm
1/29
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
30 Terms
Formation of the Mesoderm
During gastrulation, the epiblast cells that migrate through the primitive streak and occupy positions between the epiblast and endoderm will form mesoderm
Mesodermal Derivatives
The mesoderm with differentiate into the
chordamesoderm,
the paraxial mesoderm,
the intermediate mesoderm,
and lateral plate mesoderm.
Chordamesoderm
Chordamesoderm generates the prechordal plate and the notochord
Migrates through the node and is located at the midline
Also called axial mesoderm or dorsal mesoderm.
Chordamesoderm is an important signaling center
Paraxial Mesoderm
Is adjacent to the axial mesoderm and neural tube
Paraxial mesoderm in the head, or head mesoderm is unsegmented and will form skeleton, muscle, and connective tissue of face and skull, along with contributions from the cranial neural crest
Starting just posterior to the otic placode, the paraxial mesoderm is segmented into somites.
Somties are epithelial "block like", clusters of cells adjacent to the neural tube.
Somites will form muscle, bone, connective tissue, and dermis in the back of the embryo and muscles in the limbs.
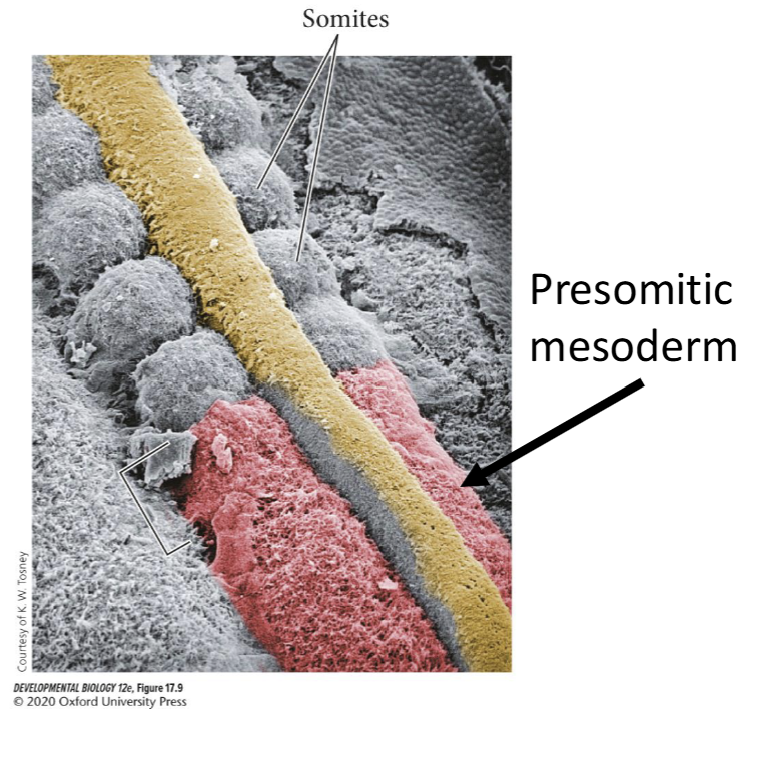
Presomitic Mesoderm
At the posterior end of the embryo, cells that have just migrated through the primitive groove are mesenchymal and unsegmented and are called premositic mesoderm
Presomitic mesoderm will unto mesenchymal to epithelial transition (MET) to form the epithelial somites
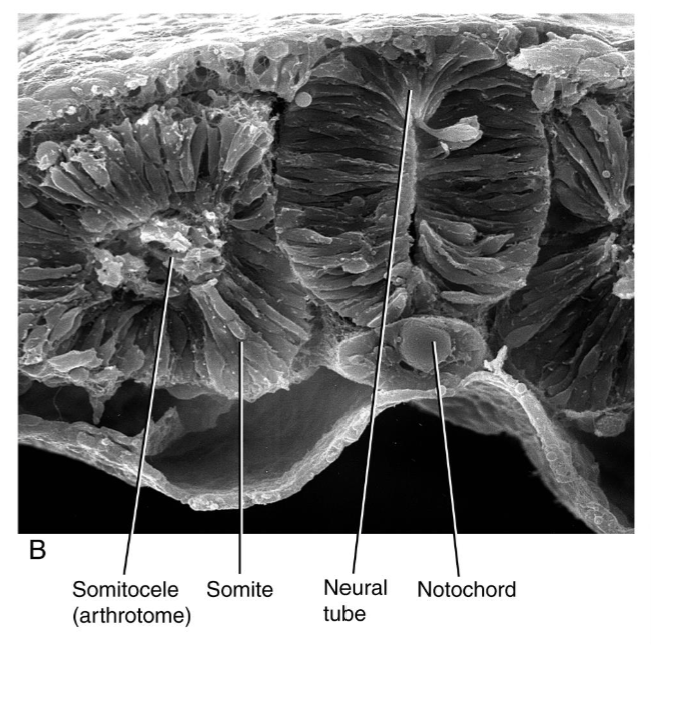
Initial Somite Structure
Newly found somites have epithelial outer layer and a mesenchymal core, the somitocele.
Somite Organization
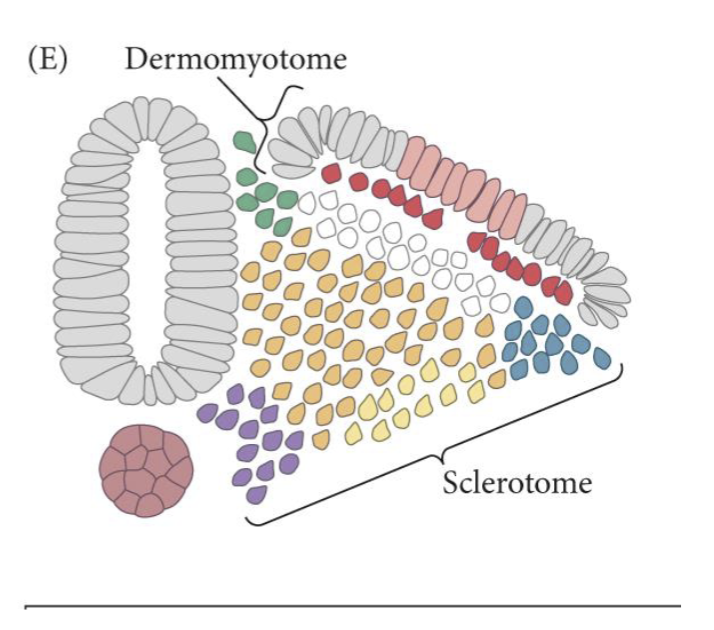
As a somite matures the ventral part will undergo an EMT, generating the sclerotome
The dorsal epithelial region is the dermomyotome
The sclerotome will form the vertebrae and associated tendons and rib cartilage
The dermomyotome will make skeletal muscle and dermis of the back
The ventral portion is the myotome and will form musculature
The dorsal surface is the dermatome and will form the dermis of the back.
Somite Differentiation
Cranial somites differentiate from presomitic mesoderm earlier than caudal somites and are developmentally older than caudal somites.
Specifying Paraxial Mesoderm
The lateral plate mesoderm expresses high level of BMP4, the notochord and the lateral portion of the somites expresses Noggin
BMP4 signaling converts mesoderm to lateral plate mesoderm, so inhibiting the BMP signal is important for specifying somites.
Brachyury, Tbx6 and Mesogenin are pioneer transcription factors for presomitic mesoderm.
The cells in the primitive streak region are bipotential, they can form either mesoderm or neural
Tbx6 mice fail to form paraxial mesoderm and the bipotential progenitors produce three neural tubes.
FGF8 and Wnt3a promote formation of the neuromesoderm progenitors (NMP) in the primitive streak region
Retinoic acid is synthesized in the somites and neural tube and promotes the differentiation of presomitic mesoderm into somites
The balance of counteracting morphogenetic gradients is critical for somite formation
Brachyury
Means short tail and describes a spontaneous mice mutant.
Heterozygotes have a short tail, and homozygotes are embryonic lethal will loss of trunk and tail mesoderm.
Brachyury has a DNA binding domain (the T-box(, which is conserved in other related transcription factors, known as Tbx.
Sacral Agenesis with Vertebral Anomalies (SAVA)
Homozygous mutations in the human T gene (TBXT)
No sacrum
Vertical clefting of all vertebral bodies.
Retinoic Acid as a Morphogen
Retinoic acid is not a protein, but a small lipophilic molecule derived from vitamin A
Receptors (RAR and RXR) are inside the cell, and when bound by retinoic acid act as transcription factors.
We can't visualize where in an embryo RA is, so we look for the enzymes, like Raldh, which produce retinoic acid, and Cyp26b, which degrades retinoic acid.
Somite Identity
Hox genes are expressed in overlapping domains in the paraxial mesoderm.
The cranial limit of expression for some Hax genes correspond to changes in vertebrate identity
Cranial limit of Hox10 expression is at the boundary of thoracic and lumbar vertebrae.
Hox gene expression in the presomitic mesoderm establishes somite identity.
Changes in expression for Hox genes in the presomitic mesoderm alters somite identity.
Loss of Hox10 paralogues, which is expressed in the lumbar and sacral region, causes these vertebrae to become thoracic like with ribs
Misexpression of Haxa10 causes cranial somites to produce lumbar vertebrae
Somite Identity Continued
Hox genes at the 3' end of the cluster are expressed at more cranial locations
Hox genes at the 5' end of the cluster are expressed at more caudal locations
Temporal collinearity of Hox genes refers to the correspondence between when a Hox gene is turned on with its spatial expression in the embryo
Hox genes at the 3' end are turned on first and are expressed at more cranial positions
Hox genes at the 5' end are turned on later and are expressed at more caudal positions.
The chromatin state of the Hox clusters change with developmental time
The 3' end of the cluster is opened first, leading to early transcription
The loosened chromatin conformation progress toward the 5' end of the cluster, leading to later transcription.
Cranial to Caudal Somite Identity
As Hox genes are activated the progenitors expressing the Hox genes enter the primitive streak and migrate into the presomitic mesoderm
More cranial somites result from progenitors that enter early
Caudal somites result from progenitors that enter late.
In chick embryos, when presomitic mesoderm is transplanted from an older embryo to a younger embryo, the transplanted tissues retains its identity
Thoracic vertebrae are produced at cervical levels
The transplanted presomitic mesoderm is already determined prior to somite formation
Somitogenesis
Somitogenesis is the process of forming somites.
The anterior end of the presomitic mesoderm is organized into groups of somitomeres.
The somitomeres are generating epithelial connections that lead to somite formation.
Somites and somitomeres are numbered such that the newest somite is SI
Clock and Wavefront Model
Somitogenesis results in the regular formation of somites, such as that a new pair of somites is generated at regular intervals
This periodicity is generated by establishing a location for a boundary to form, using a wavefront, and generating a time for the boundary to form with an internal clock.
The Wavefront
The wavefront is also called the determination front, and is where the presomitic mesoderm becomes determined
Determination occurs in the presomitic mesoderm
The location of the determination front was identified by inverting chick somitomeres so that they caudal compartment was now cranial
If a somitomere is already determined, then the caudal cells will retain their caudal identity.
If a somitomere is not determined, then the caudal cells will adopt a cranial identity, conforming to their new environment
The determination front is established by counteracting gradients of FGF8 and retinoic acid
FGF8 in the tailbud keeps presomitic progenitors in an undifferentiated state
Retinoic acid promotes determination
The Wavefront Continued
Once in a region of low FGF8 the presomitic progenitors become competent to repsond to a molecular clock.
FGF8 gradient is established in part through mRNA decay
Only progenitor cells in the tailbud transcribe FGF8 as evidenced by RNA in situ hybridization for FGF8 introns, which are only in the pre-mRNA found in the nucleus
Progenitor cells in the presomitic mesoderm have the FGF8 mRNA but they are not making new FGF8 mRNA.
As progenitor cells in the presomitic mesoderm get further away from the elongating tailbud the FGF8 mRNA is degraded, reducing the concentration of secreted FGF8, leading to determination.
Retinoic acid inhibits FGF8 transcription so presomitic progenitors cannot transcribe more FGF8 mRNA.
The Clock
A molecular clock is characterized by oscillations in gene expression
A gene is turned on and then it is turned off
Communications between cells is needed to communicate the changes in gene expression between neighboring cells
Presomitic progenitors that are past the determination front will respond to the oscillating clock and begin the mesenchymal to epithelial process to generate a new somite.
Notch, Delta, and Notch transcriptional targets have oscillating expression in the presomitic mesoderm
Transcriptional targets includes Hes1, Hes7, and LFNG
Notch transcriptional targets, like Hes7, will inhibit Notch and Delta, providing a negative feedback loop.
Notch turns on its inhibitor, which shuts, Notch signaling off, leading to oscillations
The Wavefront and Clock Come Together
High levels of FGF8 prevent NOTCH from activating Mesp2
In the S-IV region at the determination front the presomitic mesoderm has less FGF8 and is competent to respond to the Notch clock
Notch turns on Mesp2, in the cranial half of the somitomere, which results in boundary formation which the neighboring caudal compartment and a new somite is formed.
Defect in Somitogenesis
Mutations in Notch signaling components like LFNG and Delts (DII3) cause defects in somitogenesis, resulting in vertebral malformations
Known as spondylocostal dysostosis in humans
MESP2 mutations cause spondylocostal dysostosis
Severe vertebral segmentation defects.
Human Somite Congenital Malformations
caudal dysgenesis is agenesis or incomplete development of the lumbar vertebrae, sacrum, and coccyx, hypoplastic lower limbs and anorectal and genitourinary dysgenesis
Associated with diabetic mothers.
Sirenomelia
Type of caudal dysgenesis where fusion of the lower extremities causes the fetus to resemble a mermaid
Cause is unknown
Sclerotome and Dermomyotome
Paracrine signals from neighboring tissues induce transcription factors that promote sclerotome and dermomyotome formation
SHH from the notochord and floorplate cause Pax1 expression in the sclerotome.
As a somite matures it undergoes an EMT on the ventral side to generate the sclerotome
The migratory cells will move to positions around the neural tube to generate the vertebrae
The epithelial cells on the dorsal side from the dermomyotome
Sclerotome Development
The migrating sclerotome cells move to different locations, based on their location
Ventromedial cells are attracted to signals coming from the notochord and will form the vertebral body
Dorsomedial cells receive a different signal and will migrate over the neural tube to form the arch and spine of the vertebrae.
Notochord Contribution
The notochord is broken up by sclerotome cells that will generate the vertebral body
The remaining notochord cells will form the nuclei pulposi, which form the gel-like mass at the center of the invertebral discs.
Resegmentation
The caudal segment of each sclerotome will separate from the cranial segment
The caudal segment will fuse with the cranial segment behind it, in a process known as resegmentation
One vertebrae is generated from parts of two different sclerotomes (somites)
The neural crest, forming the dorsal root ganglia and sensory neurons, and motor neurons axons migrate through the cranial segments to innervate muscle from the myotome.
The myotome does not perform resegmentation
The process generates muscles that are offset from the vertebrae so that the muscles can attach to two adjacent vertebrae, allowing the spine to bend.
Dermomyotome Development
The dorsal portion of the somite that becomes the dermomyotome expresses Pax6
Two different myotome regions are formed, one close to the neural tube, which will form epaxial muscles (back muscles) and one close to the lateral plate mesoderm, which will form hypaxial muscles (body wall and limb muscles)
The myotome regions form from the dorsomedial lip (epimere) and the ventrolateral lip (hypomere) of the dermomyotome
Each region produces myoblast, or muscle precursors, which will migrate under the dermatome where they will differentiate into the myotome and form epaxial and hypaxial muscles.
Central portion of the dermomyotome will form the dermotome, which generates dermal tissue of the skin in the back
Other dermal tissue is generated from lateral plate mesoderm.
Muscle Hypertrophy
Myostatin is a TGF-B family member that functions as a negative regulator of muscle growth.
Homozygous mutation in one patient resulted in a well muscled child
Mother was a professional athlete whose father and grandfather were also exceptionally strong.