giant covalent structures, metallic bonding and modern materials
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
20 Terms
structure of graphite
a giant covalent structure with…
- 2D layers of hexagonal rings with strong bonds
- delocalised electron between layers makes it conductive/helps layer bond
- weak bonds between layers
structure of diamond
3D lattice structure
each carbon atom forms 4 very strong covalent bonds
silicon dioxide structure
3D lattice structure
2 oxygen atoms for each silicon atom
properties of graphite
insoluble in water
conducts electricity
soft/slippery between layers
high melting point
properties of diamond
insoluble in water
can’t conduct electricity
high melting point
strong and lustrous
giant covalent structure
a huge network of atoms held by strong covalent bonds
metallic bonding
electrostatic attraction between positive metal ions in a regular lattice structure and a ‘sea’ of delocalised electrons (from the outer shell)
why can metals conduct electricity and heat?
the delocalised electrons are free to move and carry charge through the structure
why do metals have strong melting points
a lot of energy is needed to break strong bonds between ions/electrons
malleable/ductile
can be bent into different shapes/can be stretched (without breaking)
why are alloys harder than pure metal
pure metal is soft due to the regular arrangement, which means layers can slide over each other
alloys are harder because different size atoms disrupt arrangement/can’t slide over each other
nanoscience
study of nanoparticles 1-100 nm in size
material behave differently at the nanoscale because…
large surface area to volume ratio
means high percentage of atoms exposed at surface
therefore very reactive/effective as catalysts
structure/properties of graphENE
a single layer of graphite made of hexagonal rings of carbon atoms
very conductive and flexible→portable electronics
structure/properties of spherical fullerenes e.g. Buckminsterfullerene (bucky balls)
hollow 3D sphere made of hexagonal rings of carbon atoms
slippery→ lubricants
structure/properties of cylindrical fullerenes e.g. nanotubes
3D hollow tube of graphene made of hexagonal rings of carbon atoms
strong, light, conductive→ catalysts
polymer
a long chain molecule made of many small molecules called monomers joined together
addition polymerisation (e.g. ethene)
chemical reaction where double bonds in the monomers break to create a single bond and 2 free electrons that can join to others
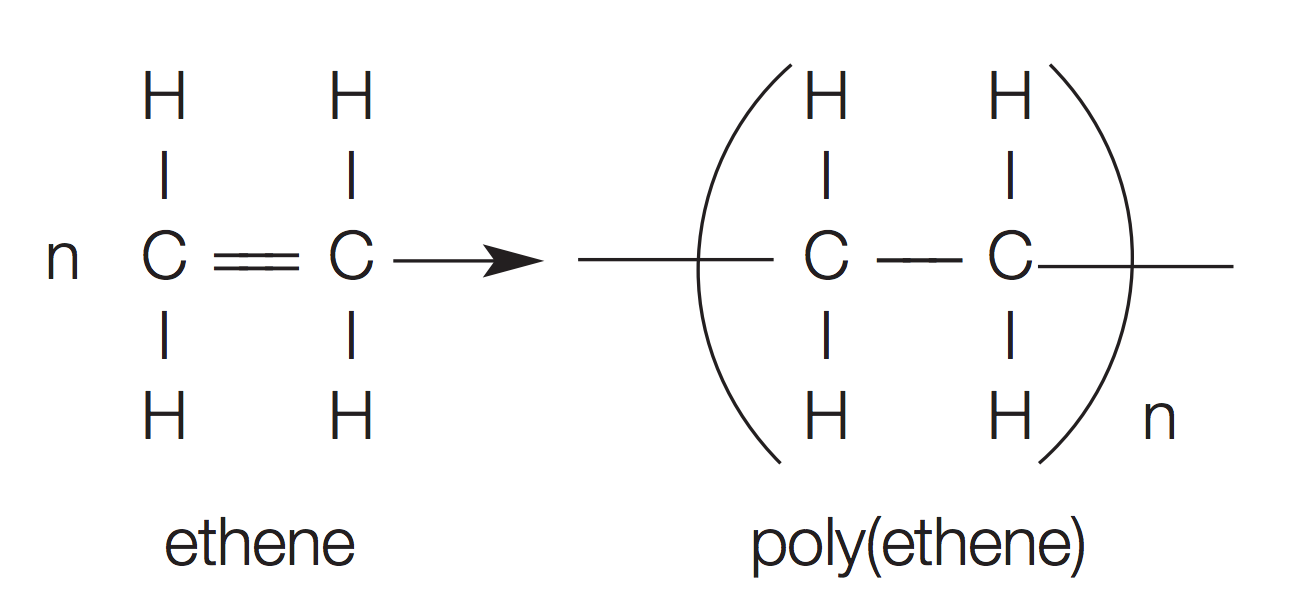
properties of plastics
soft/hard, flexible/brittle etc
depend on what monomer they are made from and conditions under which polymer was made
thermosettting vs thermosoftening plastics
thermosetting has high melting points due to cross-links between chains
thermosoftening has lower melting point due to no cross-links
