Chapter 5- The second law of thermodynamics
1/46
Earn XP
Description and Tags
Year 1 sem 1
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
47 Terms
What is the formula for a small change in entropy, dS for a reversible process?
dQrev = the reversible heat flow
T= the temperature.
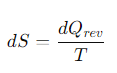
What does the First Law of Thermodynamics determine in a process?
It determines the energetic possibility of a process by ensuring energy conservation.
What does the First Law of Thermodynamics restrict?
It restricts what processes are energetically possible by conserving energy
It does not specify the direction of the process.
What does the Second Law of Thermodynamics determine?
It determines the direction in which a process occurs
It favours an increase in the entropy of the Universe.
Why can't heat spontaneously flow from a cold body to a hot body?
It would decrease the entropy of the cold object more than it increases the entropy of the hot object
This would result in a net decrease in the total entropy of the Universe, which violates the Second Law.
What ensures the spontaneous flow of heat from hot to cold bodies?
The Second Law ensures that processes spontaneously increase the total entropy of the system and surroundings
It favours heat flow from hot to cold bodies.
What happens to the entropy of the Universe when Tcold<Thot?
The entropy of the Universe increases when heat flows from a hot body to a cold body
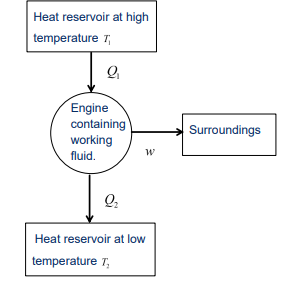
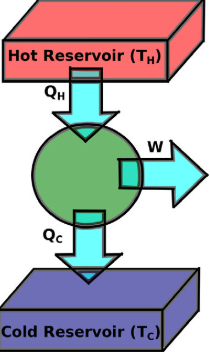
What is a heat engine?
Its a device that uses heat to generate mechanical work by carrying a working substance through a cyclic process.
What is the function of a heat engine?
In one cycle, the heat engine:
Receives heat Q1 from a high-temperature reservoir.
Emits heat Q2 to a low-temperature reservoir.
Does work w on its surroundings.
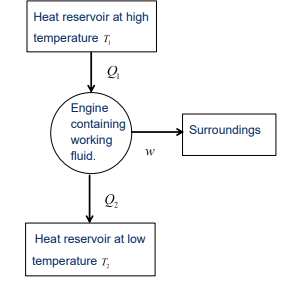
What are the sign conventions for heat and work in a heat engine?
Q1: Positive (heat absorbed by the system).
Q2: Negative (heat released by the system).
w: Negative (work done by the system on the surroundings).
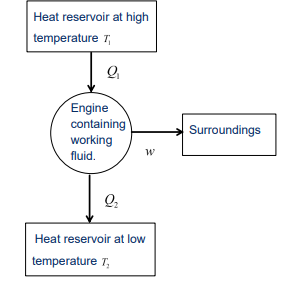
What is a Carnot heat engine?
An idealized heat engine that operates on an ideal gas through four reversible steps
Its the most efficient heat engine possible.
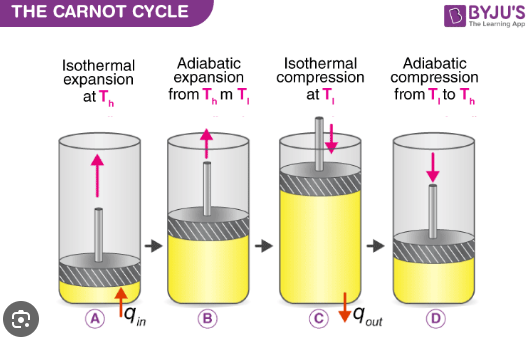
Describe the working mechanism of a Carnot heat engine.
The Carnot engine consists of a hypothetical cylinder with a frictionless piston.
It operates using an ideal gas as the working fluid.
The engine undergoes four reversible steps that return the working fluid to its initial state.
Why is the Carnot heat engine considered ideal?
Because it represents the maximum possible efficiency achievable by a heat engine operating between two temperature reservoirs.
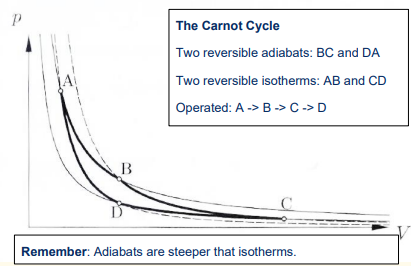
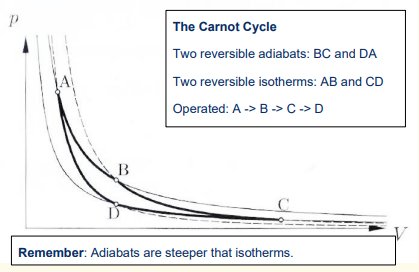
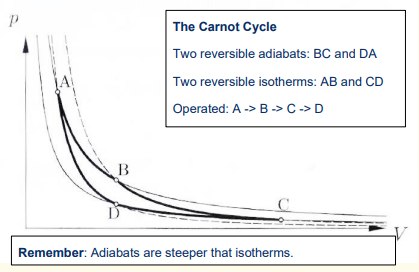
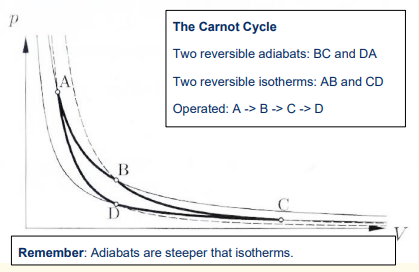
What are the 4 steps in the Carnot engine cycle?
1) Reversible isothermal expansion of the gas from State A to State B
2) Reversible adiabatic expansion of the gas from State B to State C.
3) Reversible isothermal compression of the gas from State C to State D
4) Reversible adiabatic compression of the gas from State D to State A
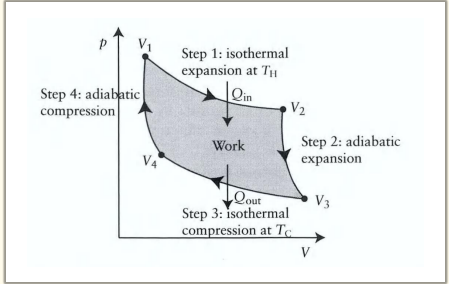
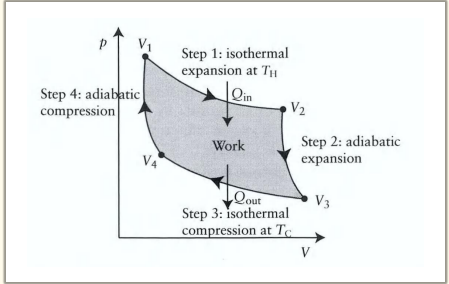
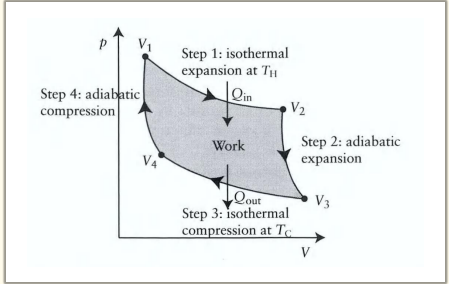
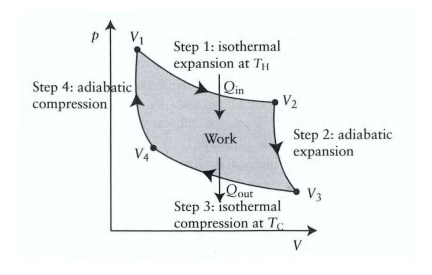
What happens during the first step of the Carnot cycle?
Reversible isothermal expansion:
The gas expands from State A to State B.
Work w1 is done by the gas on the surroundings.
Heat Q1 is absorbed from the high-temperature reservoir.
Temperature T1 of the gas remains constant.
What happens during the second step of the Carnot cycle?
Reversible adiabatic expansion:
The gas expands from State B to State C.
The piston and cylinder are thermally isolated, so no heat is gained or lost.
Work w2 is done by the gas on the surroundings.
The gas temperature drops to T2
What happens during the third step of the Carnot cycle?
Reversible isothermal compression:
The gas is compressed from State C to State D.
The surroundings do work w3 on the gas.
Heat Q2 flows out of the gas to the low-temperature reservoir.
Temperature T2 of the gas remains constant.
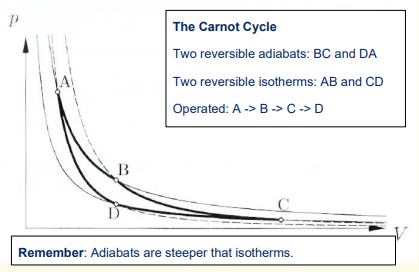
What happens during the fourth step of the Carnot cycle?
Reversible adiabatic compression:
The gas is compressed from State D to State A.
The surroundings do work w4 on the gas.
No heat is exchanged.
The gas temperature increases back to T1
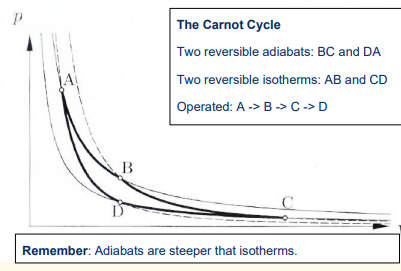
How is reversibility ensured in the first step of the Carnot cycle?
The temperature of the high-temperature reservoir is only infinitesimally higher than the gas temperature T1.
How is reversibility ensured in the third step of the Carnot cycle?
The temperature of the low-temperature reservoir is only infinitesimally lower than the gas temperature T2
How is the Carnot cycle completed?
The fourth step brings the gas back to its initial state at temperature T1 completing the cyclic process.
What is the expression for the internal energy change (ΔU) during Step 1 of the Carnot cycle (Isothermal Expansion(dT=0))?
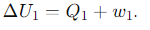
What is the expression for the internal energy change (ΔU) during Step 2 of the Carnot cycle (Adiabatic Expansion(dQ=0))?
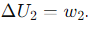
What is the expression for the internal energy change (ΔU) during Step 3 of the Carnot cycle (Isothermal Compression(dT=0))?
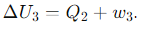
What is the expression for the internal energy change (ΔU) during Step 4 of the Carnot cycle (Adiabatic Compression(dQ=0))?
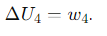
Why is the total change in internal energy over one complete Carnot cycle zero?
The gas returns to its initial state after the cycle, so ΔUcy=0
What is the total work (wcy) done by the Carnot engine in a complete cycle?
Q1 = the heat absorbed from the high-temperature reservoir
Q2 = is the heat rejected to the low-temperature reservoir.
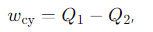
What does the first law of thermodynamics state about the Carnot cycle?
The total heat exchange is equal to the total work done in one cycle.
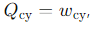
Why is wcy negative for the Carnot engine?
The engine does work on the surroundings
This makes wcy negative by convention.
How is the efficiency (ε) of a heat engine defined?
It is the ratio of the work done on the surroundings to the heat input at the higher temperature
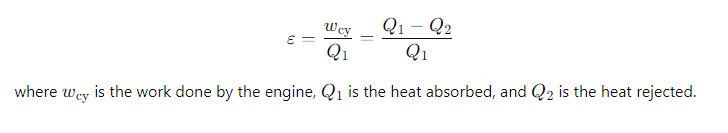
What is the simplified expression for the efficiency of a Carnot heat engine in terms of the heats?
Q1 = the heat absorbed from the hot reservoir
Q2= the heat rejected to the cold reservoir.
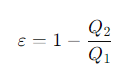
What is the range of values for the efficiency (ε) of a Carnot engine?
It is always less than 100%.
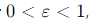
What is the goal for improving the efficiency of a Carnot engine?
We aim to reduce the magnitude of the heat rejected to the cold reservoir (Q2).
Is it possible to reduce Q2 (the heat rejected to the cold reservoir) to zero in a Carnot engine?
No
This is a limitation of the second law of thermodynamics.
What does the second law of thermodynamics state about the efficiency of heat engines?
Its impossible to construct a heat engine that is 100% efficient
This is because some heat must always be rejected to the cold reservoir.
What is the Kelvin Statement of the second law of thermodynamics?
"No process is possible whose sole result is the complete conversion of heat into work."
What is the Clausius Statement of the second law of thermodynamics?
"No process is possible whose sole result is the transfer of heat from a colder to a hotter body."
Are the Kelvin and Clausius Statements consistent with each other?
Yes. If one of them is true, then the other is also true.
What is the heat exchange for Step 1 (Isothermal Expansion) of the Carnot cycle?
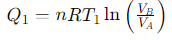
What is the relationship for temperature and volume during Step 2 (Adiabatic Compression) of the Carnot cycle?
γ= the adiabatic index.
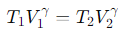
What is the heat exchange for Step 3 (Isothermal Compression) of the Carnot cycle?
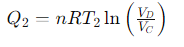
What is the relationship for temperature and volume during Step 4 (Adiabatic Compression) of the Carnot cycle?
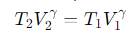
What is the key relationship between heat exchange and temperature during the Carnot cycle?
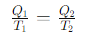
What is the thermodynamic definition of temperature?
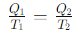
What formula expresses the efficiency (ϵ) of a Carnot engine in terms of temperatures?
T1= the temperature of the high-temperature reservoir
T2= the temperature of the low-temperature reservoir.
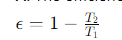
How can high efficiencies be achieved in a Carnot engine?
By minimizing the ratio T2/T1:
Make T1 as high as possible and T2 close to room temperature.