Biology - Chapter 3 Enzymes
1/26
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
27 Terms
Define enzymes.
A protein produced by living organisms that act as biological catalysts in biochemical reactions by reducing activation energy.
*It’s a catalyst because it speeds up the rate of reactions but remains unchanged at the end.
Describe enzymes and their functions.
They are globular proteins
They have a specific shape due to the 3 dimensional coiling of the enzyme molecule.
Hydrophilic R grps are on the outside of the molecule - making it soluble (in water and cytoplasm)
They are involved in all metabolic reactions and are therefore essential for life.
What are the 2 types of enzymes
Intracellular (those that work inside cells)
Extracellular (those that are secreted by the cells and catalyse reactions that take place outside the cell).
What are the 2 types of mode-of-action of enzymes?
lock-and-key hypothesis
induced-fit hypothesis
Explain the lock-and-key hypothesis.
All enzymes have a surface (called the binding site) on which substrates can bind.
The shape of the active site and the substrate is complementary.
They fit exactly to one another (like a lock and key) to form an enzyme-substrate complex.
The substrate is held in place by temporary bonds between the R-groups and the substrate molecule.
Each enzyme can bind to only one type of substrate molecule, because the shape of the active site is only complementary to that shape of the substrate molecule - shows specificity
Definition of lock-and-key hypothesis:
A hypothesis of the mode of action of enzymes, where the active site of an enzyme and the substrate molecule have complementary shapes, and it fits exactly onto the site - it shows specificity.
Explain the induced-fit hypothesis.
Similar to lock-and-key hypothesis, but instead of the active site and substrate having exactly complementary shapes:
The enzyme active site (and sometimes the substrate), can change its shape slightly as the substrate enters the active site. This allows the active site and the substrate to have a more perfect fit and therefore a more efficient catalysis.
Definition of the induced-fit hypothesis:
A hypothesis for the mode of action of enzymes, where the active site and substrate have complementary shapes, but not an exact fit. Instead, the active site (and sometimes the substrate) can change its shape slightly to ensure a perfect fit. The enzymes still show specificity though.
What happens after an enzyme-substrate complex is formed.
The reaction takes place, where either:
A substrate is split into 2/more smaller molecules (catabolism)
2/more substrates are joined together to make a larger molecule (anabolism)
When the reaction is complete an enzyme-product complex is temporarily formed.
The products then leave the active site, and the enzyme remains unchanged and so is available to receive another molecule.
How does lysozyme work?
It’s a natural defence against bacteria:
It breaks the polysaccharide chains that form the cell wall of the bacteria.
How do enzymes increase the rate of reaction?
Rate of reaction must be fast, otherwise chemical reactions in and out of cells occur so slowly that life cannot exist.
All these reactions require some form of energy to convert substrate → product.
Activation energy (energy required to start a chemical reaction: to convert substrate → product, enzymes lower this energy)
Energy can be provided in the form of heat.
To avoid this, enzymes lowers the Ea required for reactions.
They do this by holding the substrate(s) in a way that is easier for them to react.
Reactions take place more rapidly at lower temps. than they originally would.
How to measure the rate of reaction of an enzyme catalysed reactions.
Measuring the rate of formation of a product.
For example: catalase
Catalyses the reaction of the breakdown of H2O2 (toxic) into H2 and O2.
It must be gotten rid of quickly - oxygen is collected to measure rate of reaction.
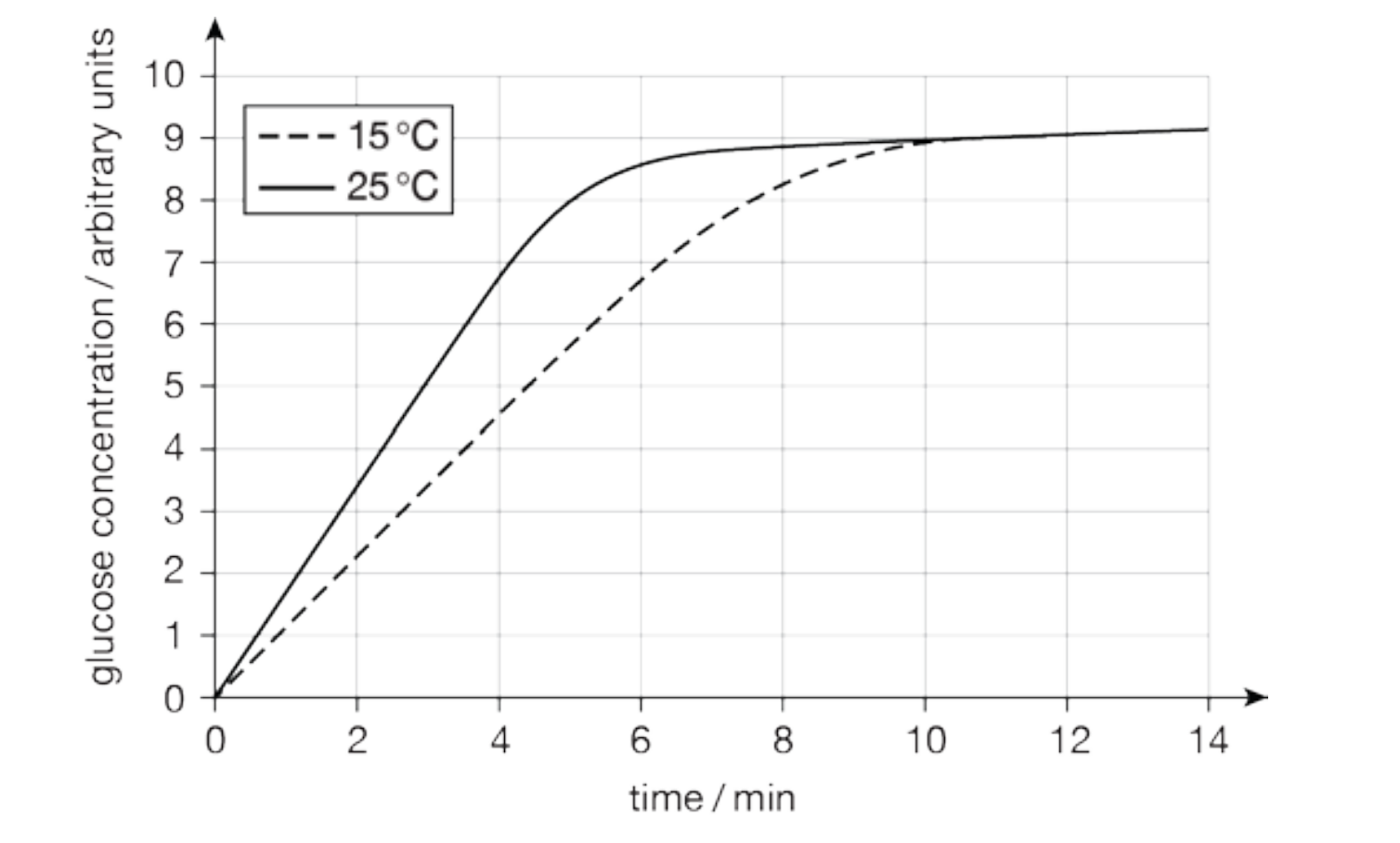
Describe the rate of reaction, and the production of product from a reaction.
The reaction starts very quickly. As soon as the substrate and enzyme is mixed, products are released.
A large amount of product is collected in the first minute, and as the reaction continues the rate at which product is produced slows down.
The reaction gets slower and slower until it stops completely.
Explain the rate of reaction, and the production of product from a reaction.
When an enzyme and substrate are first mixed, there are a large number of substrate molecules.
Almost every enzyme therefore has a substrate bound to its active site.
The rate of reaction depends on the:
conc. of enzymes.
how long it takes for an to convert the substrate into the product, release it and bind to another substrate molecule.
As more substrate is converted to product, there is less substrate for the enzyme to bind to.
Enzymes are waiting for substrate molecules to collide into their active sites - reaction slows down (as fewer and fewer substrate molecules are left), until it stops.
The curve is steepest at the beginning (initial rate of reaction)
How does a colorimeter work and how can it be used to measure the progress of a reaction.
It measures the colour of a solution by measuring the absorption of different wavelengths of light.
If the method for measuring process, involves colour change - colorimeter can be used.
It quantitatively measures the colour of light (provides values which can be graphed).
The greater the absorption of wavelengths of light by a solution, the more concentrated the substance is.
What are the 2 main factors that affects enzyme activity?
Temperature
pH
Explain the effect of temperature on enzyme activity.
At low temperatures the rate of reaction is slower.
The substrate molecules and enzymes move more slowly due to them having lower KE.
They therefore collide less frequently, so substrate molecules are less likely to collide and bind to the active site of an enzyme - rate of reaction decreases.
At higher temperatures, the rate of reaction is higher.
The substrate molecules and enzymes are moving more faster due to higher KE
They therefore collide more frequently, where substrate molecules more likely to collide and bind to the active site of an enzyme.
Substrate molecules also collide with more energy, which makes it more easier for bonds to break or form for the reactions to occur.
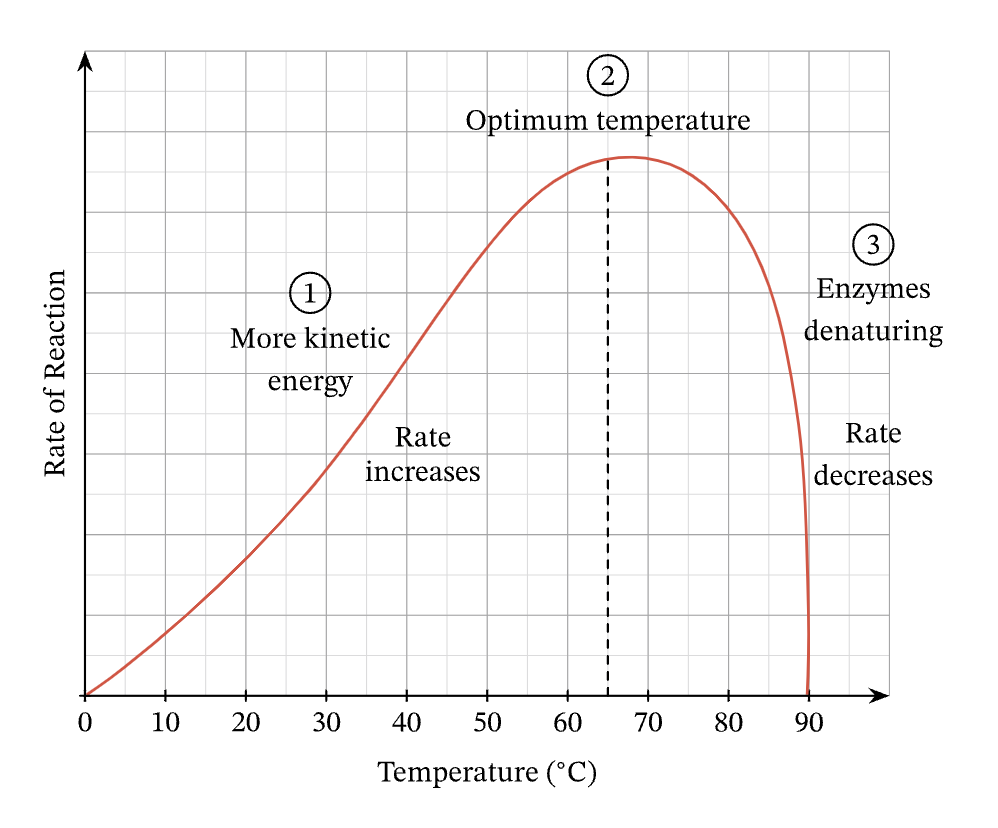
What happens to the rate of reaction after the optimum temperature has been passed? Explain.
After passing the optimum temperature the rate of reaction slows down.
This is because the enzyme begins to vibrate so much that bonds (especially H-bonds) starts to break.
This affects the 3-dimensional structure of the enzyme, as the bonds which hold the enzyme in its precise shape starts to break.
The active site begins to lose its shape and is ‘denatured’.
At first, the substrates fit less well to the active site - slowing the rate of reaction.
But after a while, the substrate cannot fit into the active site at all, and the reaction stops.
What is optimum temperature and pH.
The temperature/pH at which the rate of reaction is at its highest.
Explain the effect of pH on enzyme activity.
Most enzymes work fastest at neutral pH 7.
Pepsin (protease) found in acidic conditions of the stomach has an optimum pH of 1.5
pH is a measure of the conc. of H+ ions.
The higher the conc. of H+ ions, the lower the pH of the solution.
Since they are +vely charged, they are attracted to -vely charged ions and repelled by +vely charged ions.
H+ ions therefore interact with any charged R-grps of amino acids in the protein.
This can break ionic bonds between R-groups which affects the 3-dimensional structure of the enzyme.
The shape of the active site may change and reduce the chance of substrate molecules binding to it.
It causes denaturation.
Explain the effect of the concentration of enzyme on rate of reaction.
When individually measuring the rate of reaction for each [enzyme], similar curves are seen, where initially the reaction is fast but then slows down.
To compare the rate of reaction of each individual [enzyme] - best to compare the initial rate:
Because amount of substrate once reaction goes on varies, as substrate is converted to product at diff rates for diff [enzyme].
Only at start is [substrate] the same
This way we know any change in rate is due to only the differences in [enzyme] and not [substrate]
Initial rate is directly proportional to the enzyme concentration.
If more enzymes are present, there is more active sites for the substrate to bind to.
*As long as plenty of substrate available, rate of reaction increases linearly with [enzyme].
![<ul><li><p>When individually measuring the rate of reaction for each [enzyme], similar curves are seen, where initially the reaction is fast but then slows down.</p></li><li><p>To compare the rate of reaction of each individual [enzyme] - best to compare the initial rate:</p><ul><li><p>Because amount of substrate once reaction goes on varies, as substrate is converted to product at diff rates for diff [enzyme]. </p></li><li><p>Only at start is [substrate] the same</p></li><li><p>This way we know any change in rate is due to only the differences in [enzyme] and not [substrate]</p></li></ul></li><li><p>Initial rate is directly proportional to the enzyme concentration. </p><ul><li><p>If more enzymes are present, there is more active sites for the substrate to bind to. </p></li></ul></li></ul><p>*As long as plenty of substrate available, rate of reaction increases linearly with [enzyme].</p>](https://knowt-user-attachments.s3.amazonaws.com/3e79160e-7c9b-492d-b4e2-92f68d8a395e.png)
Explain the effect of the concentration of substrate on rate of reaction.
Initial rate plotted against [substrate].
As [substrate] increases, the rate of reaction also increases:
More substrate molecules = more often will one enter the active site
If you keep increasing the [substrate], keeping [enzyme] constant - there comes a point when every active site becomes full.
Since enzymes cannot work faster, substrate molecules are effectively queuing up for a free active site.
The enzyme is working at its maximum possible rate - Vmax
The enzyme is saturated with substrate.
it is a very useful indicator for the efficiency of an enzyme.
![<ul><li><p>Initial rate plotted against [substrate].</p></li><li><p>As [substrate] increases, the rate of reaction also increases:</p><ul><li><p>More substrate molecules = more often will one enter the active site</p></li></ul></li><li><p>If you keep increasing the [substrate], keeping [enzyme] constant - there comes a point when every active site becomes full.</p></li><li><p>Since enzymes cannot work faster, substrate molecules are effectively queuing up for a free active site.</p></li><li><p>The enzyme is working at its maximum possible rate - V<sub>max</sub></p></li><li><p>The enzyme is saturated with substrate.</p></li><li><p>it is a very useful indicator for the efficiency of an enzyme.</p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/efa81efb-a551-4ebe-b153-0f6c80e320cb.png)
Define Vmax.
The theoretical maximum possible rate of an enzyme-controlled reaction, when all active sites are occupied.
Explain ‘comparing enzyme affinities’
Affinity is a measure of strength of attraction between 2 things. High affinity = strong attraction.
Affinity is a measure of strength of attraction between an enzyme and its substrate.
The greater affinity an enzyme has for its substrate - the faster the reaction.
more likely a product will be formed when a substrate enters the active site of an enzyme.
If low affinity, the substrate may leave the active site before it reacts.
Explain what Km is, how it it calculated and what it is used for.
When initial rate plotted against [substrate], you get a curve.
This curve never flattens out in practice - it only does so in theory at infinite [substrate].
Cannot read value of Vmax from graphs
½ Vmax can be calculated - exactly half the maximum velocity (indicates how fast enzymes work)
Can also find the [substrate] resulting in ½ Vmax - [substrate] at which exactly half of the enzyme active sites are filled with substrate.
Km is the [substrate] that causes ½ Vmax.
Full form = Michaelis-Menten constant
The [substrate] at which an enzyme works at half the maximum rate of reaction (½ Vmax ). used as a measure of efficiency of an enzyme.
The lower the value of Km - the higher the efficiency of the enzyme:
the higher the affinity between an enzyme and its substrate, the lower the [substrate] required to reach Vmax.
![<ul><li><p>When initial rate plotted against [substrate], you get a curve.</p></li><li><p>This curve never flattens out in practice - it only does so in theory at infinite [substrate]. </p></li><li><p>Cannot read value of V<sub>max</sub> from graphs</p><ul><li><p>½ V<sub>max </sub>can be calculated - exactly half the maximum velocity (indicates how fast enzymes work)</p></li><li><p>Can also find the [substrate] resulting in ½ V<sub>max</sub> - [substrate] at which exactly half of the enzyme active sites are filled with substrate.</p></li></ul></li><li><p>K<sub>m</sub> is the [substrate] that causes ½ V<sub>max</sub>.</p></li><li><p>Full form = Michaelis-Menten constant</p><p>The [substrate] at which an enzyme works at half the maximum rate of reaction (½ V<sub>max</sub> ). used as a measure of efficiency of an enzyme. </p></li><li><p>The lower the value of K<sub>m</sub> - the higher the efficiency of the enzyme:</p><ul><li><p>the higher the affinity between an enzyme and its substrate, the lower the [substrate] required to reach V<sub>max. </sub></p></li></ul></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/ea748a8c-2299-4397-bca5-76110b1753ee.png)
Define and explain competitive inhibitors.
When a substance reduces the rate of an enzyme-controlled reaction, by competing with the substrate for the active site of the enzyme. The degree of inhibition is decreased when [substrate] increases. Degree of inhibition increases when [inhibitor] increases.
Explanation:
molecule similar in shape to substrate entering active site - inhibits enzyme function.
if inhibitor binds briefly to site and then comes out - there is competition between the substrate and inhibitor.
more substrate than inhibitor - enzyme function is unaffected.
if less substrate than inhibitor/ substrate conc. falls, then there are less available active sites to collide into
Enzyme function inhibited.
However it is reversible.
Define and explain non-competitive inhibitors.
A substance that reduces the activity of an enzyme, but increasing the concentration of the substrate doesn’t reduce the degree of inhibition. Many of these inhibitors bind to areas of the enzyme that aren’t the active site.
Explanation:
While the inhibitor is bound to the enzyme, it affects the normal arrangement of hydrogen bonds and hydrophobic interactions that holds the enzyme in its specific shape in 3 dimensions
This results in the distortion of the shape of the active site, inhibiting the ability of the substrate entering the active site.
While the inhibitor is attached to the enzyme, the enzyme function is blocked.
Inhibition can be harmful, but in some situations - essential:
metabolic reactions must be controlled - no enzyme can be allowed to work without stopping at a point (then more product is constantly made)
The end-product of a chain of reactions is used as a non-competitive, reversible inhibitor.
end product, inhibits the enzyme at the start of the chain: the enzyme iss gradually slowed as the end product increases.
End product can lose its attachment to the enzyme (reversible) - if the product is used somewhere else, the enzyme becomes active once more and makes more product.
The regulation of the amount of end product = end product inhibition
Define immobilised enzymes.
enzymes fixed to a surface or trapped in beads of agar gel.
Why are immobilised enzymes useful.
enzymes are used in industrial processes
they are very expensive and must be recycled:
when fixed to a surface/trapped in beads, it is prevented from diffusing freely in solution.
And if the product being made is a solution, the solution will be contaminated with the enzyme, as it is a very difficult job to get the enzyme back again.
immobilised enzymes are more tolerant of temp. and pH changes than in solution.
maybe because they are held firmly in shape in the beads in which they are embedded (doesn’t denature easily)
maybe because the parts of the enzymes that are embedded in the beads aren’t exposed to temp/pH changes.