introduction to a level chemistry
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
12 Terms
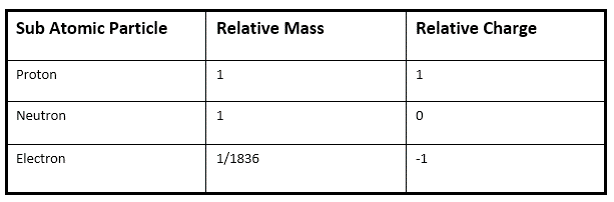
What are the masses and charges of sub atomic particles?
What are sub-shells in terms of energy levels and which energy level contains what sub-shells?
Each energy level of an is split into sub-levels called "sub-shells". The first 4 sub-shells are called: "s", "p" , “d” and “f”.
the first energy level contains the “s” sub shell only. The second energy level contains an “s” and “p” sub shell The third energy level contains an “s” , “p” and a “d” sub shell. The fourth energy level contains An “s”, “p” ,“d” and “f” sub shell.
How many electrons does each sub-shell hold
“s” holds up to 2 electrons
“p” holds up to 6 electrons
“d” holds up to 10 electrons
“f” holds up to 14 electrons
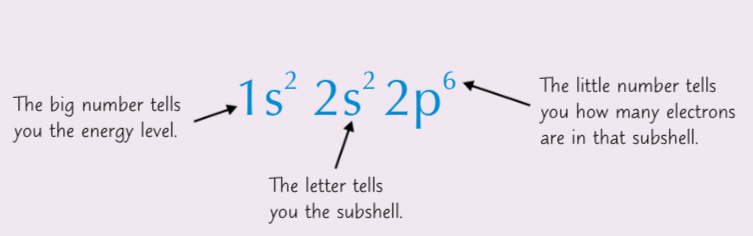
How to write the electron configuration of an atom.
The big number represents the energy level. The letter represents the sub-shell. The number to the power of the letter represents the number of electrons in that subshell.
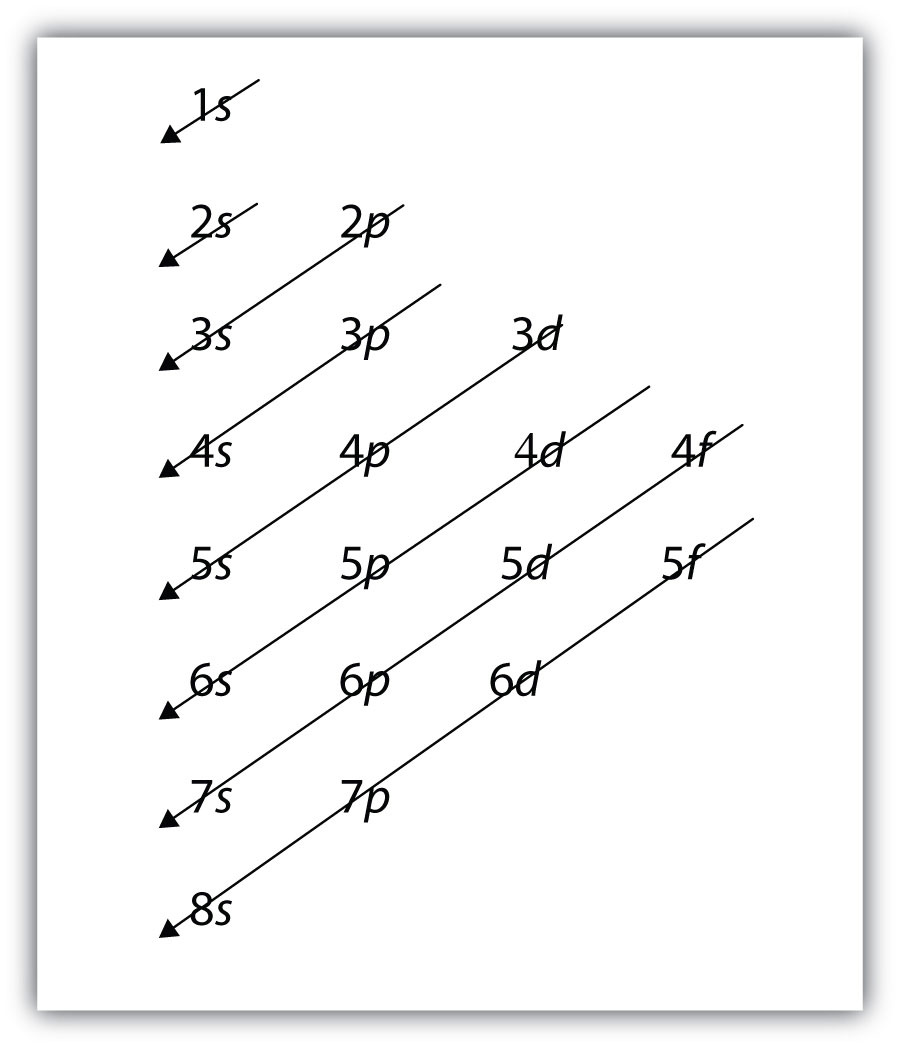
How sub shells get filled (in order)
What is the exception when writing the electron configuration of transition metals and why is this?
“D” sub-shells can only be half filled or completely filled. This is because they are more stable in this state compared to partially filled. In order to become half or fully filled, they must borrow electrons from the nearest “s” subshell
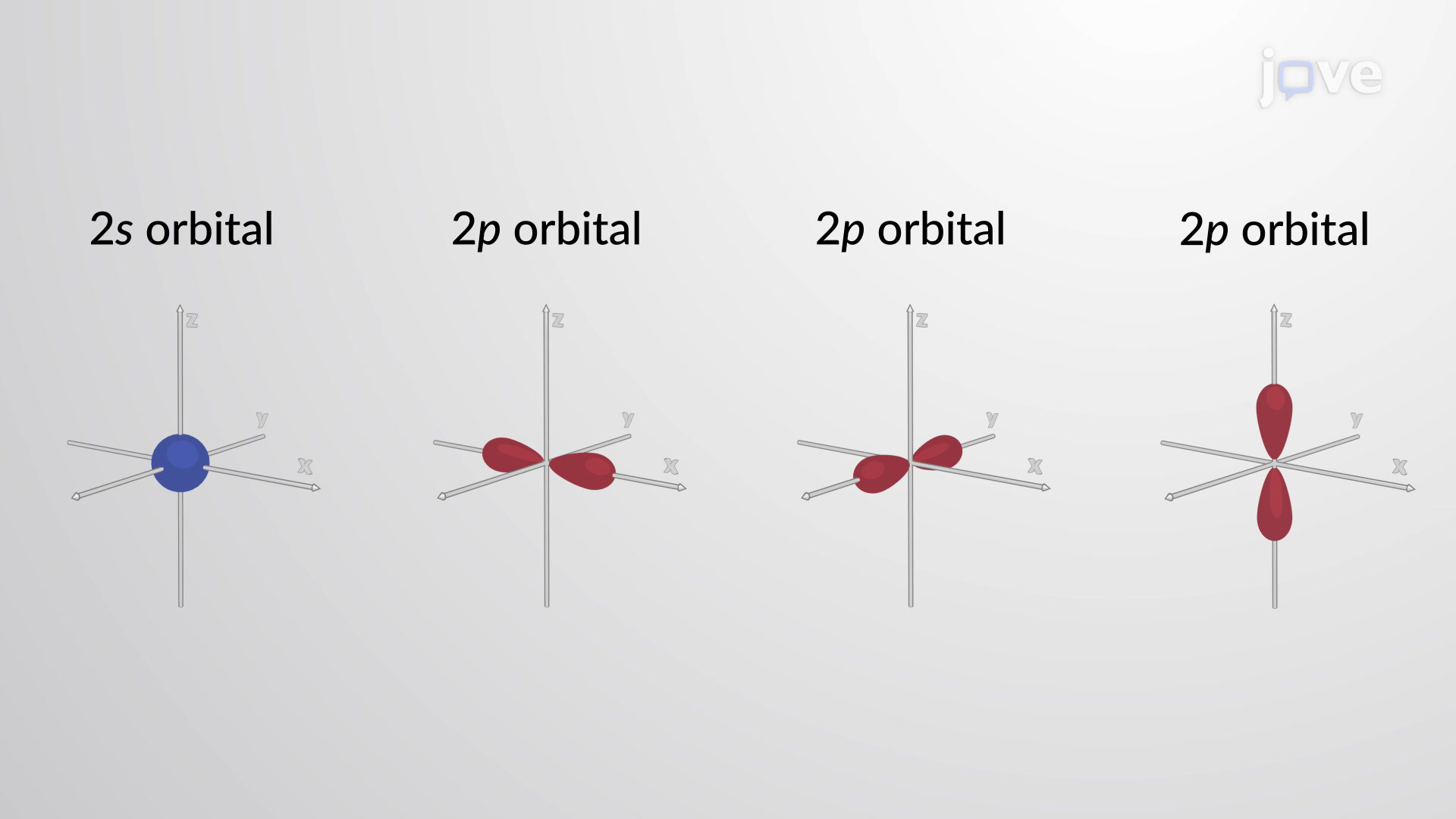
What are orbitals in terms of electrons.
Electrons are in orbitals, an orbital is a 3d region around an atoms nucleus where there is a high probability of finding an atom.
Each sub-shell contains a specific number of orbitals, each capable of holding two electrons.
The s sub-shell has one orbital (total of 2 electrons).
The p sub-shell has three orbitals (total of 6 electrons).
The d sub-shell has five orbitals (total of 10 electrons).
The f sub-shell has seven orbitals (total of 14 electrons).
How do the electrons in these orbital regions spin and why?
Opposite spin: The two electrons in the same orbital must have opposite spins (one "spin up" and one "spin down") This is to minimise replusion
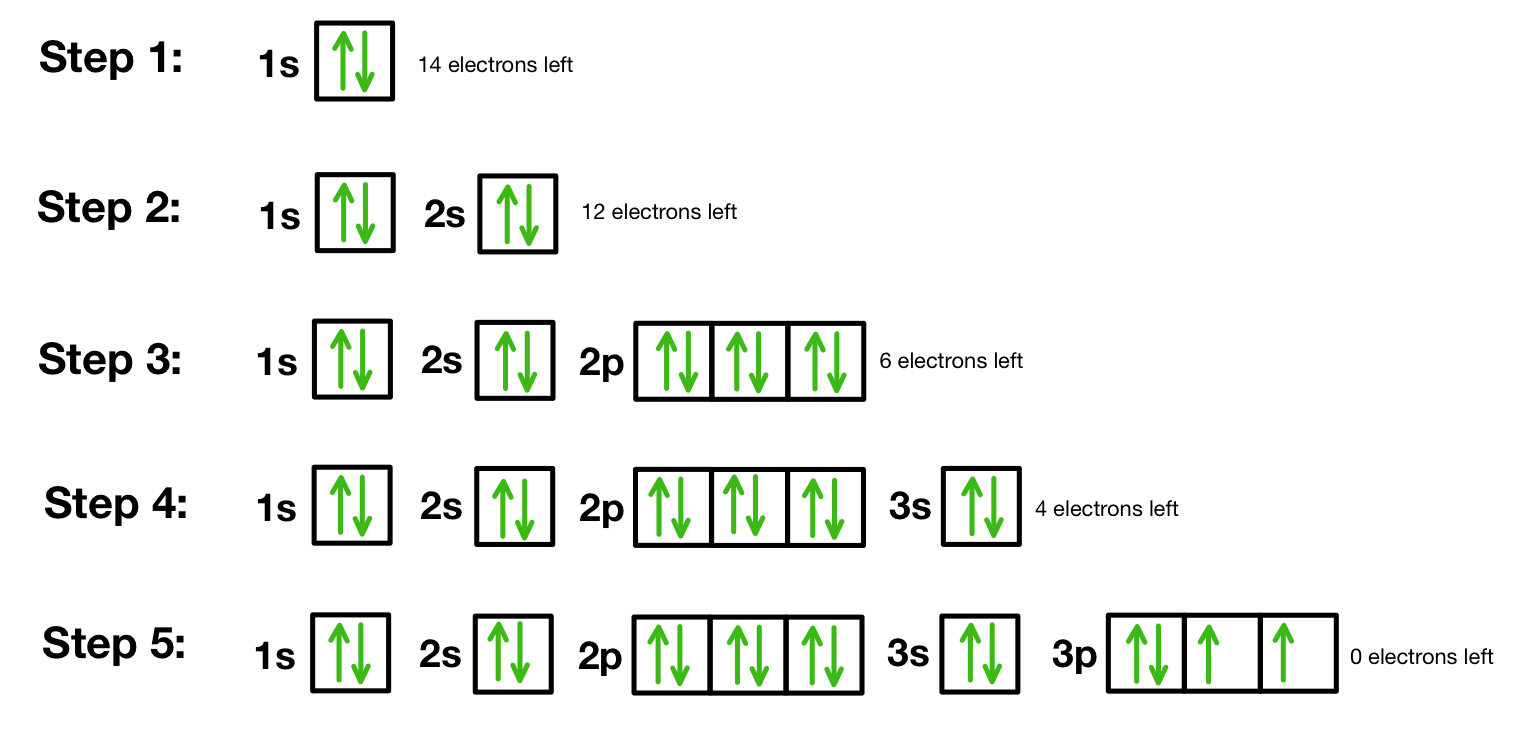
How to draw orbital diagrams
Fill the orbitals in order of increasing energy. The order is: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, etc.
Each orbital can hold a maximum of two electrons. When an orbital has two electrons, they must have opposite spins, which you show with one arrow pointing up (↑) and the other pointing down (↓).
When you have orbitals of the same energy level (like the three 2p orbitals), fill each one with a single electron first, all with the same spin direction. Then, if you have more electrons, go back and add the second electron to each orbital with the opposite spin. This minimises electron-electron repulsion.
Describe and explain the s-block and the p-block of the periodic table?
Groups 1 and 2 of the periodic table (the alkali metals) are called the s-block elements. Their outer electrons are "s" sub-shell energy levels, meaning they can accommodate up to 2 electrons. Group 3 and 0 are p-block elements, there outer electrons are in energy levels called "p" sub-shells, meaning they can accommodate up to 6 electrons. The only exception in group 0 is helium, which is an s-block element
What is ionisation energy?
The energy you need to remove the first outer electron is called the first ionisation energy. Full definition: the first ionisation energy is the energy needed to remove 1 electron from each atom in 1 mole of gaseous atoms. The ionisation energy is always a positive number as it needs to be "added" to overcome the attraction between the negative electron and the positively charged nucleus. The lower the ionisation energy, the easier it is to remove the outer electron and form an ion
1. nuclear charge: the more protons there are in the nucleus, the stronger the positive charge is , forming a stronger attraction with electrons.
2.Distance from the nucleus: Attraction decreases with distance, so an electron closer to the nucleus will be more strongly attracted than one further away
3. Shielding: electrons in shells closer to the nucleus can stop the outer electrons from feeling the full force of the nuclear charge. The inner electrons are said to "shield" the outer electrons from the nucleus, so more inner electrons = more shielding = weaker attraction between outer electrons and nucleus = lower ionisation energy