Cellular Adaptation and Cell Necrosis – Review Flashcards
1/42
Earn XP
Description and Tags
A comprehensive set of question-and-answer flashcards covering definitions, mechanisms, examples, and key distinctions related to cellular adaptation and necrosis, suitable for exam review.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
43 Terms
What is cellular adaptation?
Prolonged exposure of cells to adverse or exaggerated stimuli that evokes reversible changes in cells, tissues, or organs.
Name the six basic forms of cellular adaptation.
Atrophy, Hypertrophy, Hyperplasia, Metaplasia, Dysplasia, Anaplasia.
How do physiologic and pathologic adaptations differ?
Physiologic adaptations are normal, controlled responses to stimuli (e.g., pregnancy); pathologic adaptations occur in disease states and can be harmful (e.g., ventricular hypertrophy from hypertension).
Define atrophy.
Decrease in the size of a tissue, organ, or the entire body caused by shrinkage of individual cells.
Give three examples of physiologic atrophy.
Thymic involution with age; ovaries, uterus, and breasts after menopause; osteoporosis in bones
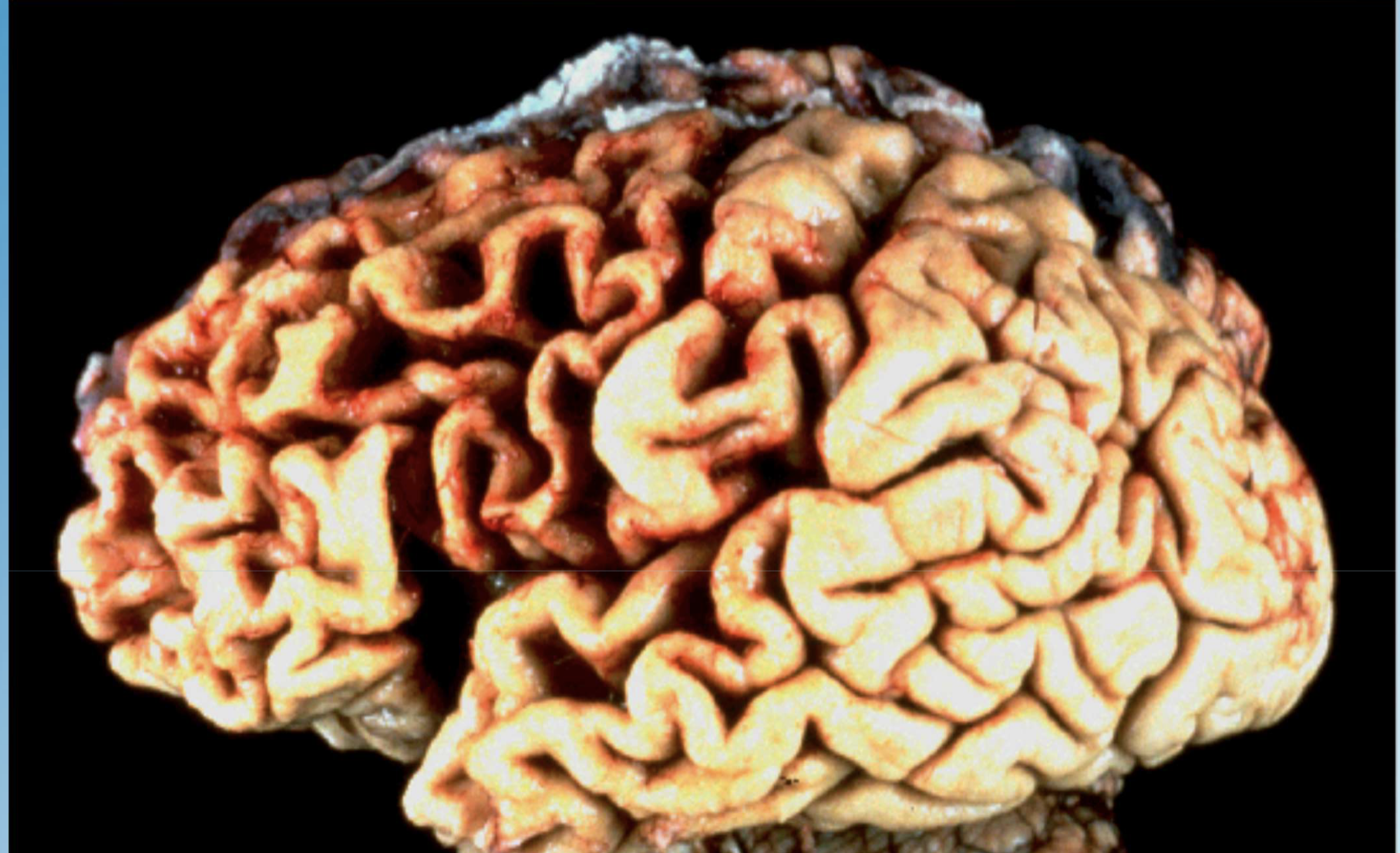
Give three examples of pathologic atrophy.
Ischemic kidney atrophy from atherosclerosis; testicular atrophy; generalized cerebral atrophy in Alzheimer disease.
Define hypertrophy.
Increase in the size of tissues or organs due to enlargement of individual cells without an increase in cell number.
Provide an example of physiologic hypertrophy.
Enlargement of skeletal muscles in body builders due to weight training.
Provide an example of pathologic hypertrophy.
Concentric left-ventricular hypertrophy resulting from chronic hypertension.
Define hyperplasia.
Adaptive increase in the number of cells causing enlargement of tissues or organs.
Give two clinical examples of hyperplasia.
Endometrial hyperplasia from excess estrogen; hyperplastic polyps of the colon or stomach.
Can hyperplasia and hypertrophy occur together? Give an example.
Yes. Uterine smooth muscle enlarges during pregnancy by both hypertrophy and hyperplasia; benign prostatic hyperplasia (BPH) shows both processes.
Define metaplasia.
An adaptive change of one cell type by another better suited to the environment.
Name two common examples of metaplasia.
Squamous metaplasia of bronchial epithelium in smokers; intestinal (glandular) metaplasia of the lower esophagus in Barrett esophagus.
Is metaplasia reversible?
Yes, if the irritating stimulus is removed.
To what more serious change can metaplasia progress?
It may progress to dysplasia.
Define dysplasia.
Disordered, precancerous growth of tissue resulting from chronic irritation or infection.
What is the relationship between dysplasia and cancer?
Dysplasia is considered a precancerous condition that can evolve into invasive carcinoma if untreated.
What is the best-known example of dysplasia in clinical practice?
Cervical intraepithelial neoplasia (CIN) detected on PAP smears.
Which virus is strongly associated with cervical dysplasia and cancer?
Human papillomavirus (HPV). Often goes undetected because of lack of symptoms
Define anaplasia.
Undifferentiated, uncontrolled cell growth that is the hallmark of malignant transformation.
List four synonyms for anaplasia.
Malignancy, Carcinoma, Cancer, Neoplasm.
Give three tumor examples that exhibit anaplasia.
Squamous cell carcinoma of the cervix, lung cancer, malignant melanoma (melanocarcinoma).
Name five microscopic hallmarks of anaplasia.
1) Marked cellular and nuclear pleomorphism; 2) Irregular, hyperchromatic nuclei; 3) High nuclear-to-cytoplasmic ratio (~1:1); 4) Prominent nucleoli; 5) Numerous abnormal mitotic figures.
Define necrosis.
Death of cells or tissues within a living organism accompanied by inflammation.
How does necrosis differ from autolysis?
Necrosis occurs in living tissue with inflammation; autolysis is self-digestion of tissues after death, without inflammation.
List the four main types of necrosis.
Coagulative, Liquefactive, Caseous, Fat necrosis.
Describe coagulative necrosis.
Most common type; cell proteins denature, preserving cell outlines with granular cytoplasm.
Give a classic example of coagulative necrosis.
Myocardial infarction of the heart; wedge-shaped infarcts of kidney or spleen.
Describe liquefactive necrosis.
Dead cells are digested by enzymes, producing a soft, liquid mass.
Give two settings where liquefactive necrosis is seen.
Brain infarcts (stroke); bacterial abscesses in lung or liver.
Describe caseous necrosis.
A form of coagulative necrosis producing a soft, cheesy, yellow material.
Give a classic disease featuring caseous necrosis.
Pulmonary tuberculosis with Ghon complex; also seen in fungal infections like histoplasmosis.
Describe fat necrosis.
Specialized liquefactive necrosis of fat caused by lipolytic enzymes, leading to saponification with calcium.
Give a typical example of fat necrosis.
Peripancreatic fat necrosis after acute pancreatitis or pancreatic trauma.
Differentiate wet gangrene from dry gangrene.
Wet gangrene: necrotic tissue secondarily infected and liquefied by bacteria; Dry gangrene: mummified, black, dried-out necrotic tissue without infection.
Name two underlying conditions predisposing to gangrene.
Atherosclerotic ischemia of limbs/intestine; diabetes mellitus.
Define dystrophic calcification.
Macroscopic deposition of calcium salts in dead or dying tissues despite normal serum calcium levels.
List four examples of dystrophic calcification.
1) Atherosclerotic coronary arteries; 2) Calcified aortic or mitral valves (stenosis); 3) Calcifications around breast cancers seen on mammography; 4) Periventricular brain calcifications in congenital toxoplasmosis.
Define metastatic calcification.
Calcium deposition in normal tissues secondary to hypercalcemia or disturbed calcium metabolism.
Give three conditions associated with metastatic calcification.
Hyperparathyroidism, vitamin D toxicity, chronic renal failure; may produce calcium stones in kidney, gallbladder, or bladder.
How do hyperplasia and hypertrophy fundamentally differ?
Hyperplasia increases cell number; hypertrophy enlarges existing cells—both can enlarge an organ but through different mechanisms.
Describe the difference between dystrophic and metastatic calcification?
Dystrophic calcification occurs in damaged or necrotic tissues with normal serum calcium levels, while metastatic calcification results from elevated calcium levels due to systemic conditions.