Chapter 12 | Solutions
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
21 Terms
Henry’s law
c = kP
c is the concentration (M) of the dissolved gas
P is the pressure of the gas over the solution
k is a constant for each gas (mol/L●atm) that depends only on temperature
Molarity (M)
moles of solute / liters of solution
Mass percentage of solute
(grams of solute / grams of solution) x 100%
Molality (m)
moles of solute / kg of solvent
Raoult’s Law
Psolution < P°solvent
Psolution = XsolventP°solvent
Boiling-point elevation
ΔTb = mKb
Freezing-point depression
ΔTf = mKf
ΔTf = Tf pure−Tf solution
Mole fraction
X = moles of solute / total moles of solution
Osmotic Pressure
π = MRT
π = osmotic pressure
M = molarity
R = gas constant = 0.082 (L • atm/ mol • K)
T = temperature in K
Colligative Properties of Electrolyte Solutions
Boiling-Point Elevation: ΔTb = iKbm
Freezing-Point Depression: ΔTf = -iKfm
Osmotic Pressure (π): π = iMRT
Van’t Hoff Factor
i = total number of ions separated from an ionic compound
Solution
A homogenous mixture of 2 or more substances
Solute
The substance (s) present in the smaller amount (s)
Solvent
The substance present in the larger amount
Solubility
The amount that dissolves in a given quantity of solvent at a given temperature
“like dissolves like”
Two substances with similar intermolecular forces are likely to be soluble in each other
Miscible
Fluids that mix with or dissolve in each other in all proportions
Immiscible
Fluids that do not dissolve in each other
Solubility of gases
More soluble at high temperatures and low pressure
Solubility of solids
Solubility increases at higher temperatures
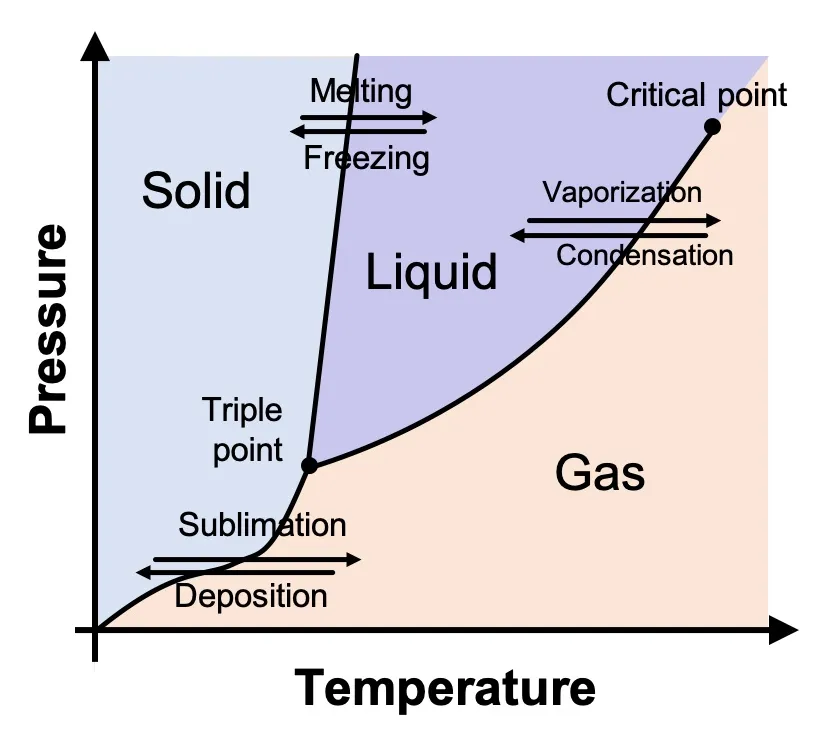
Phase diagram