Chemistry II Lesson 1: Dot Structures and VSEPR Part I
1/44
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
45 Terms
True or false? In the second period of the periodic table, it is possible to exceed 8 electrons in the valence shell, but an atom may not have less than 8.
False. In the second period of the periodic table, it is possible to have less than 8 electrons in the valence shell, but not more than 8 (as seen in this image)
This is called the "octet rule"

CRB True or false? Any molecule with an odd number of valence electrons cannot give a full octet to each of its component atoms.
True. Any molecule with an odd number of valence electrons cannot give a full octet to each of its component atoms.
CRB An atom X is neutral and has 3 valence electrons. Which of the following atoms could X be?
(See periodic table here: http://www.chem.qmul.ac.uk/iupac/AtWt/table.gif )
(A) Ga
(B) P
(C) Sr
(D) Ge
(A) Ga
Gallium is in group 3A, so it will have 3 valence electrons when neutrally charged.

The first step in drawing dot structures is to determine the number of valence electrons for each atom involved. How many valence electrons does Silicon have?
See periodic table here: http://www.chem.qmul.ac.uk/iupac/AtWt/table.gif
Silicon has 4 valence electrons.

How many valence electrons does Fluorine have?
Fluorine has 7 valence electrons.

Next you need to add up the total number of valence electrons. How many total valence electrons does SiF4 have?
(7x4) + 4 = 32
The next step is to decide on the central atom. Which should be the central atom for SiF4 and why?
The central atom for SiF4 should be Si because it is the least electronegative as compared to F.
Draw the dot structure for SiF4.

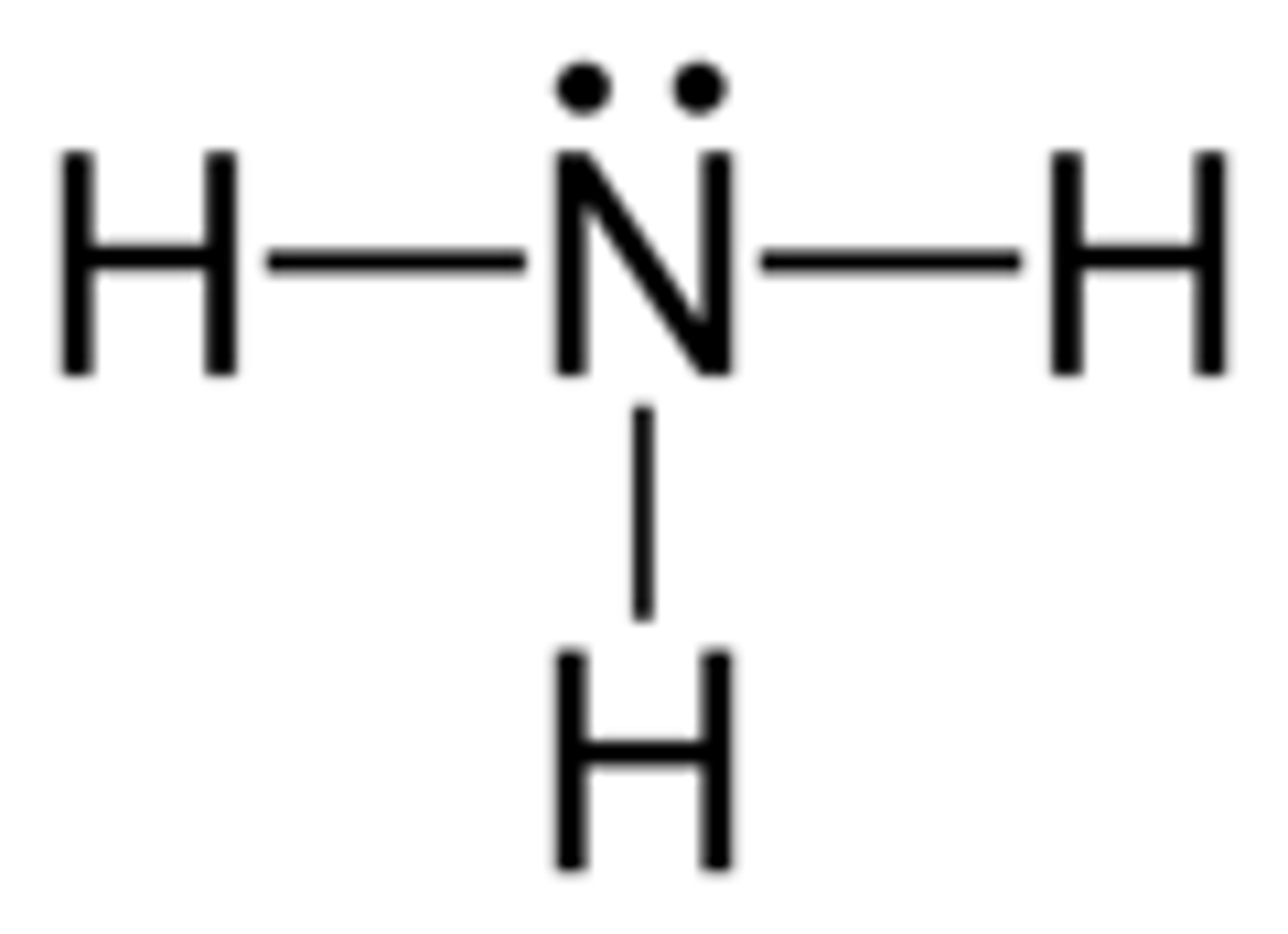
Draw the dot structure for NH3.
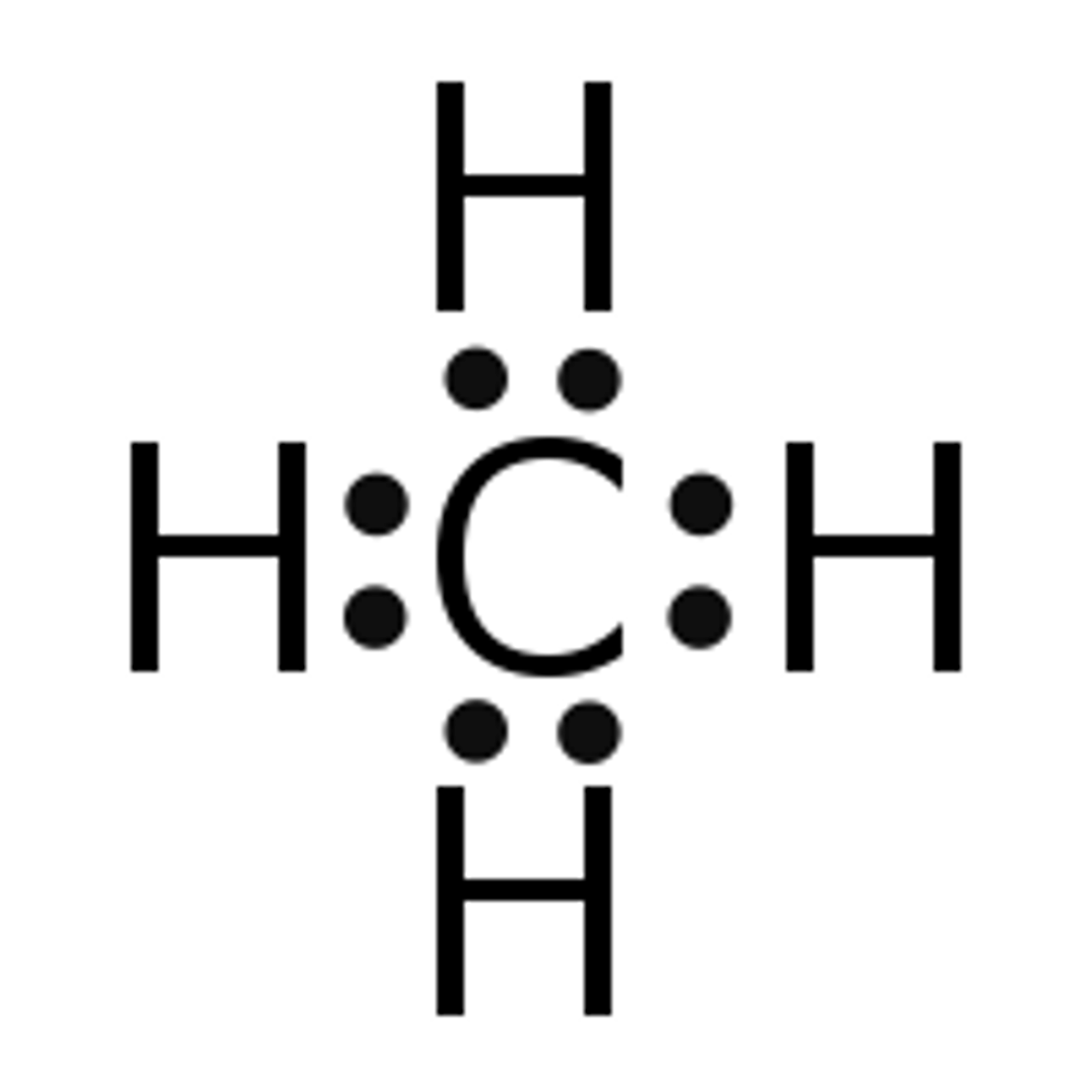
Draw the dot structure for CH4.
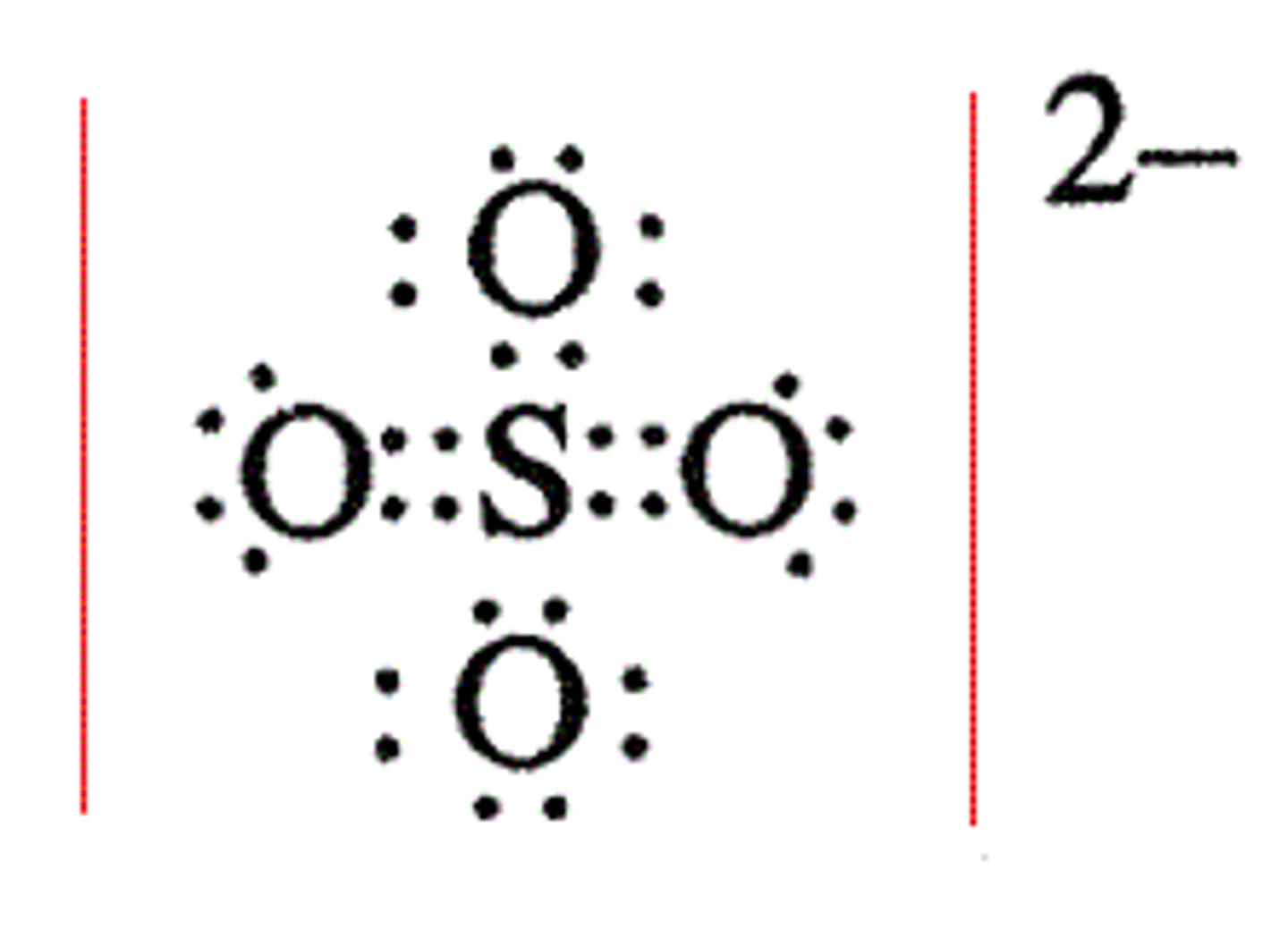
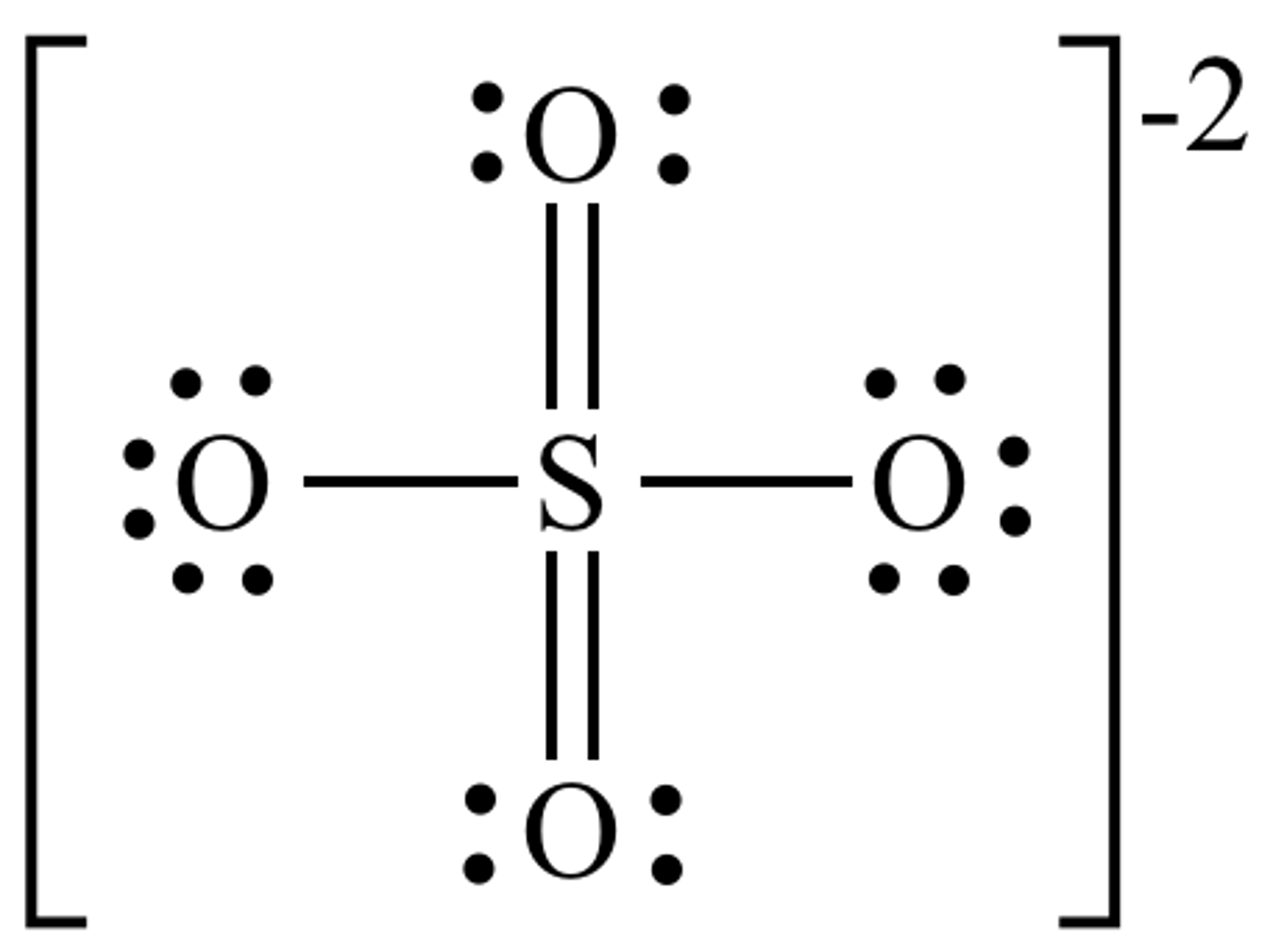
Draw the dot structure for Sulfate.
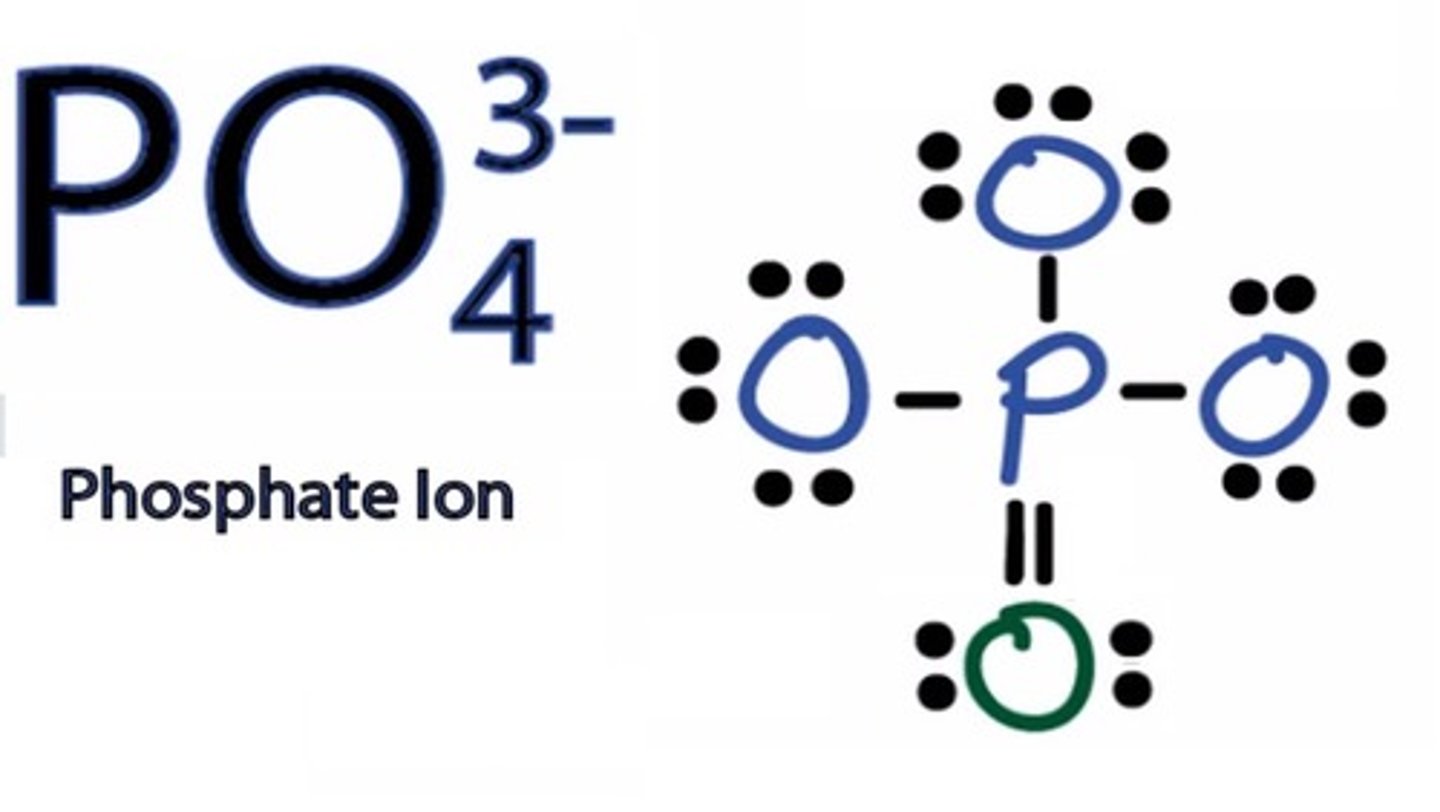
Draw the dot structure for Phosphate.
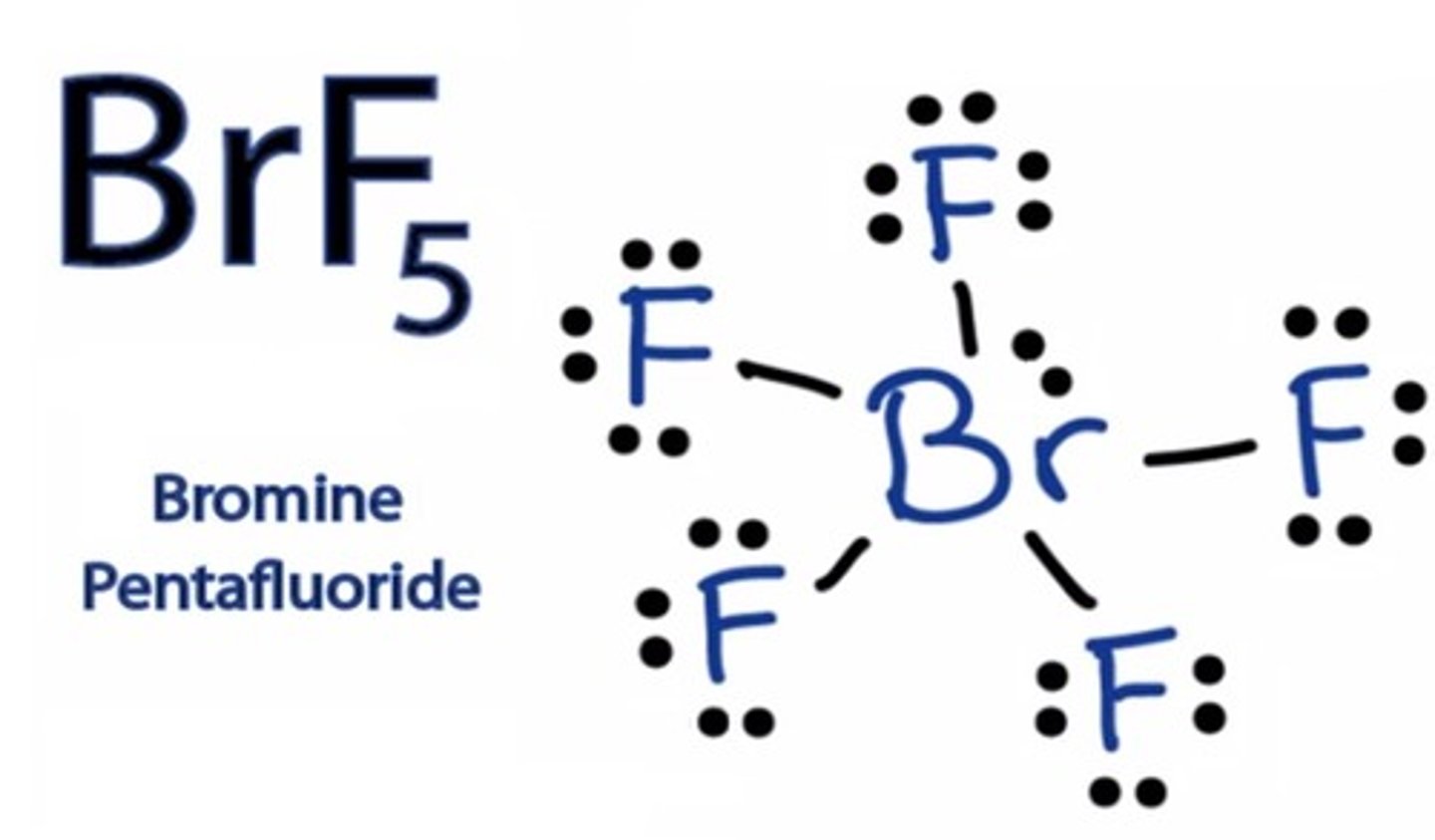
Draw the dot structure for BrF5.
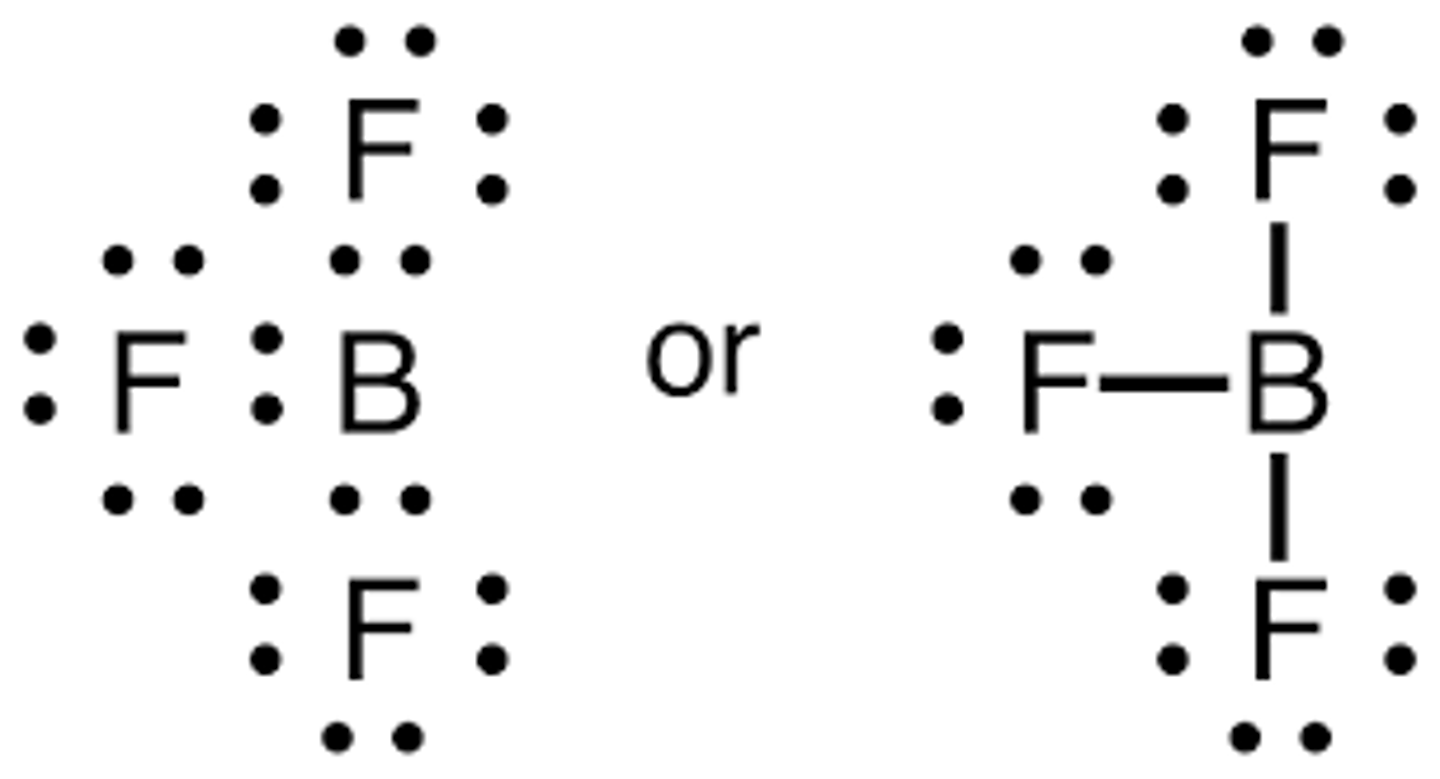
Draw the dot structure for BF3.
How do you assign formal charges to atoms in a molecule?
Formal Charge = (# of valence electrons in free atom) - [Lone pair electrons + 1/2 shared electrons]
![<p>Formal Charge = (# of valence electrons in free atom) - [Lone pair electrons + 1/2 shared electrons]</p>](https://knowt-user-attachments.s3.amazonaws.com/dfefe997-1f89-420c-a8b5-f49b69b10b46.jpg)
CRB In a hypothetical molecule, a Nitrogen has two sets of lone pair electrons and two covalent bonds. What would its formal charge be?
(A) -2
(B) -1
(C) 0
(D) +1
(B) -1
Formal Charge = 5 - [4 + 4/2] = -1
CRB True or false? In calculating formal charges, a double bond is treated exactly the same as a single bond when doing calculations.
False. In calculating formal charges, a double bond is treated exactly the same as TWO single bonds when doing calculations.
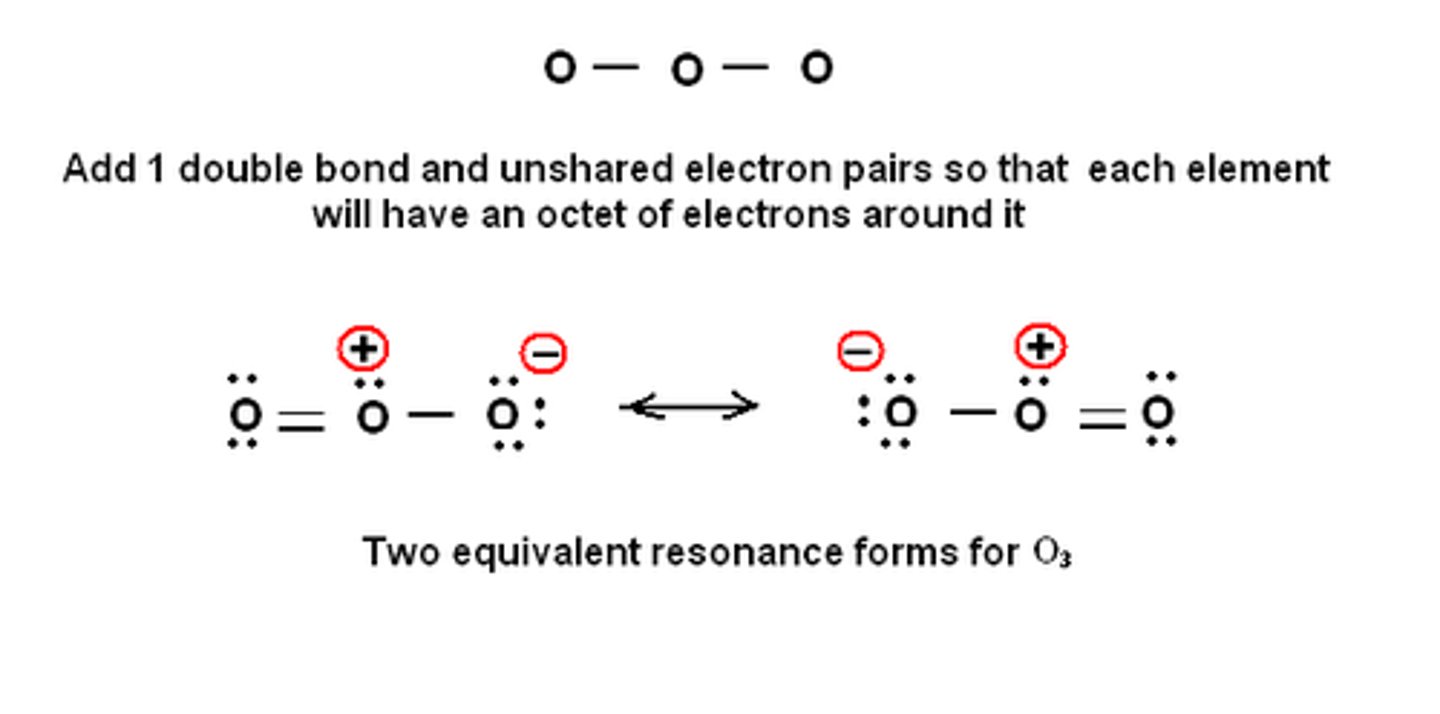
Draw the dot structure for O3, being sure to assign formal charges as needed.
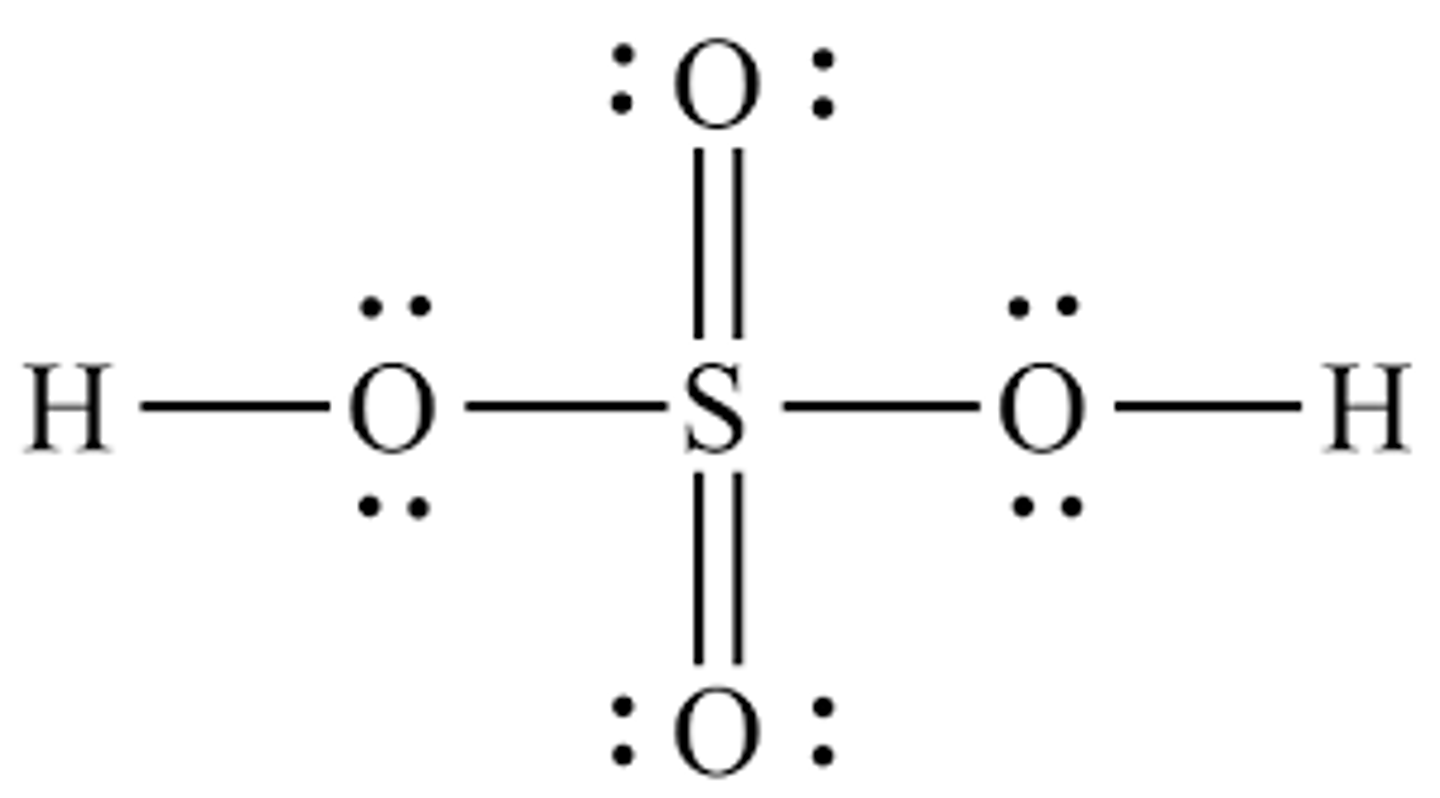
Draw the dot structure for Sulfuric Acid, being sure to assign formal charges as needed.
Draw the dot structure for Nitrate, being sure to assign formal charges as needed.
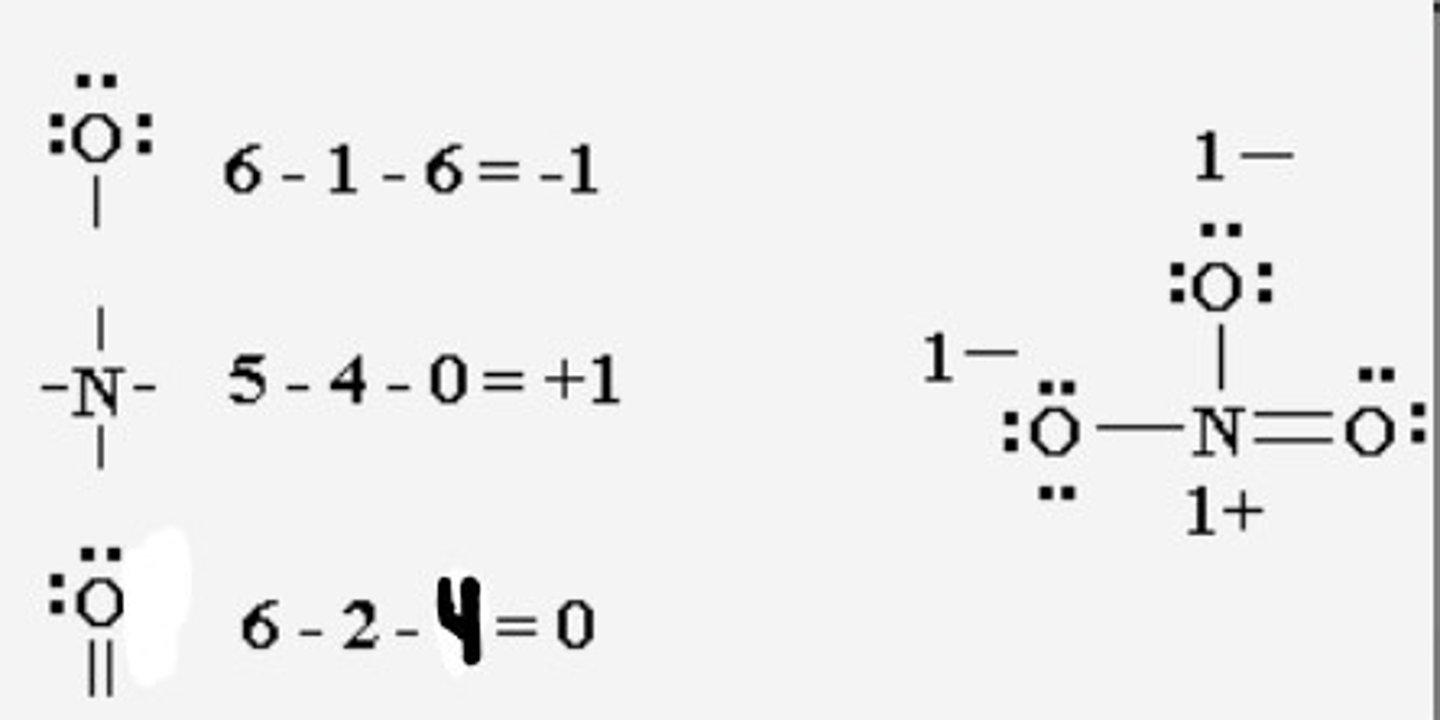
Nitrate has several resonance structures. What does this mean?
(A) The extra electrons are shared between all three bonds all at the same time
(B) One-third of Nitrate in nature will be found with one resonance structure, one-third with another, and one-third with another
(C) Every so often, the resonance structure of Nitrate will switch from one to the other
(D) Most of Nitrate will be found in the most stable form, the other two forms may be observed on a rare occasion
(A) The extra electrons are shared between all three bonds all at the same time
Resonance structures simply help us represent Nitrate. In reality, the extra electrons are shared between all three bonds all at the same time.
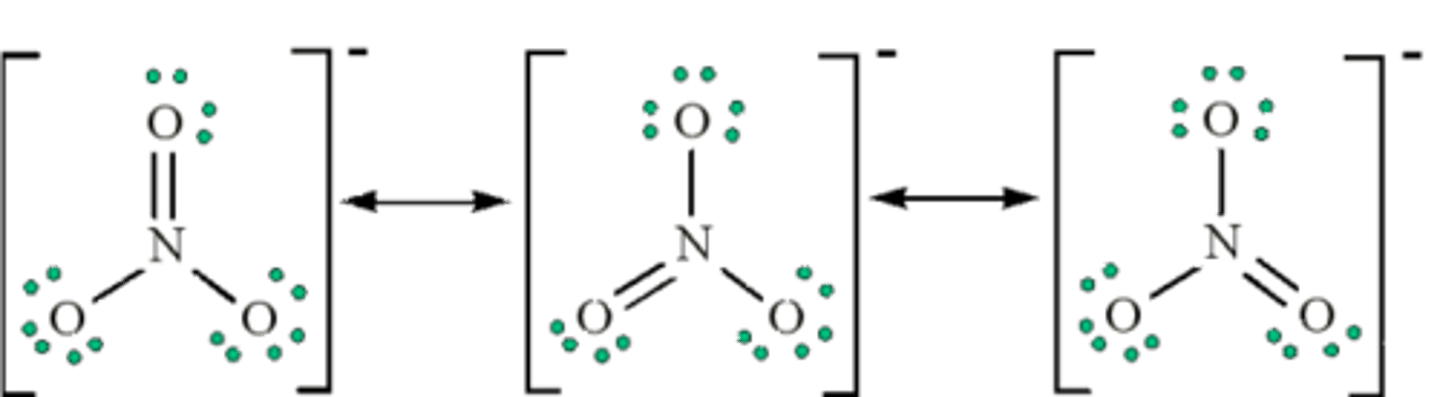
Now, draw the three resonance structures for Nitrate.
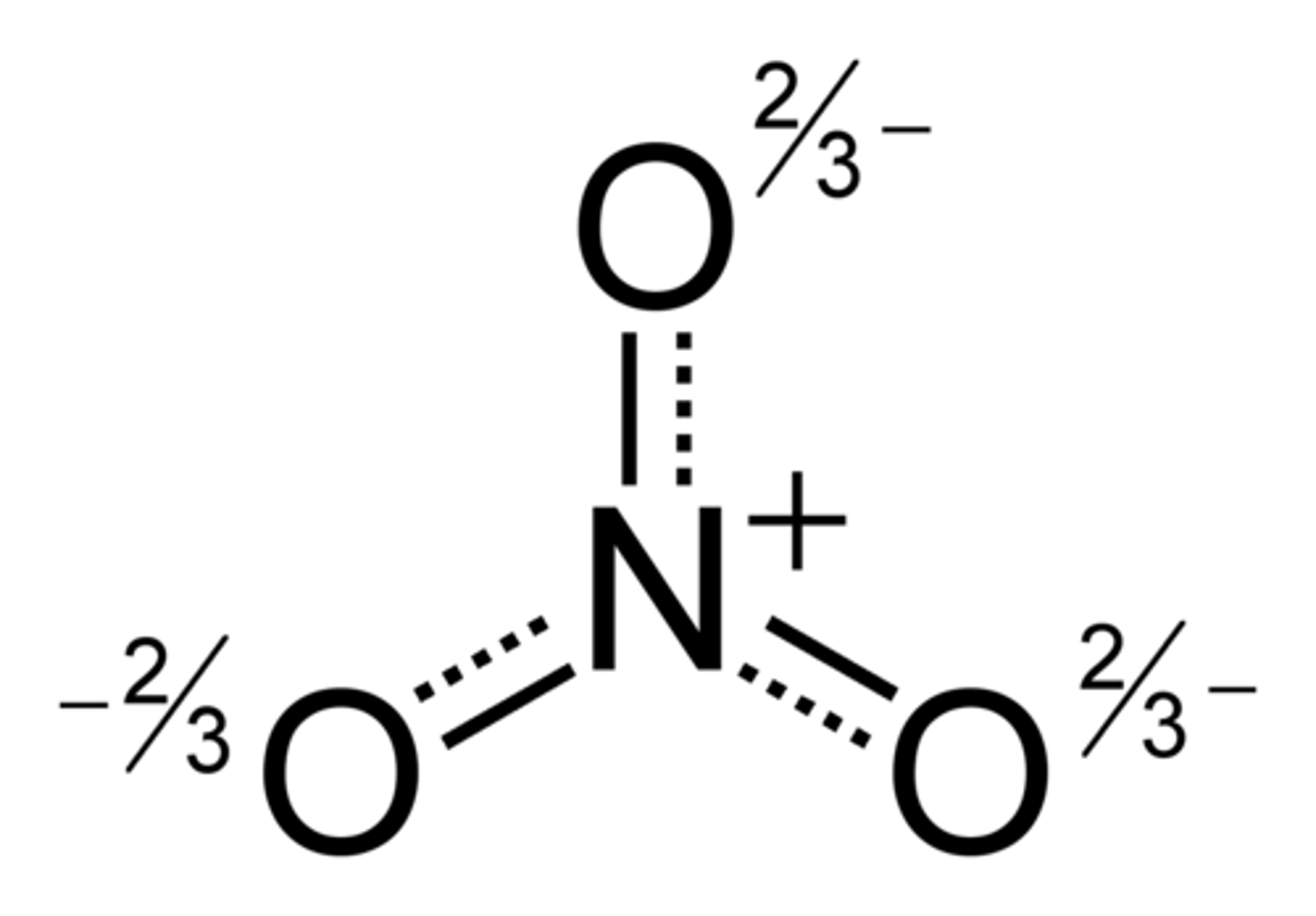
CRB In order to avoid having to draw multiple resonance structures, a resonance hybrid can be drawn. Which of the following are correct descriptions of a resonance hybrid?
I. Resonance hybrids often depict partial charges.
II. For where a double or single bond may exist, a dotted line can be used to represent the second bond.
III. Resonance hybrids are considered the "averages" of the possible resonance structures.
(A) I and II only
(B) I and III only
(C) II and III only
(D) I, II and III
(D) I, II and III
Each of the following are a correct description of resonance hybrids:
I. Resonance hybrids often depict partial charges.
II. For where a double or single bond may exist, a dotted line can be used to represent the second bond.
III. Resonance hybrids are considered the "averages" of the possible resonance structures.
CRB One of the main uses of calculating formal charges is to determine the relative stability of resonance structures. Which of the following scenarios will make a resonance structure more stable?
I. Having small or no formal charges (as opposed to larger formal charges).
II. Having more separation between opposite charges (as opposed to less separation between positive charges).
III. Having negative formal charges placed on more electronegative atoms (as opposed to negative formal charges on less electronegative atoms).
(A) I only
(B) I and II only
(C) I and III only
(D) I, II and III
(C) I and III only
Three general guidlines for having more stable resonance structures are:
I. Having small or no formal charges (as opposed to larger formal charges).
II. Having LESS separation between opposite charges (as opposed to MORE separation between positive charges).
III. Having negative formal charges placed on more electronegative atoms (as opposed to negative formal charges on less electronegative atoms).
CRB Which of the following is NOT one of the main tenants of VSEPR theory?
(A) Since electrons repel one another, electron pairs attempt to move as far apart as possible.
(B) Lone pairs will repel slightly more than bonding pairs, decreasing the bond angles by about 2.5 degrees per lone pair.
(C) The electron and molecular geometries will always be the same, since they are both predicted by electron placement.
(D) For any central atom with a given number of lone pairs plus bonding pairs, the electron geometry will be identical.
(C) The electron and molecular geometries will always be the same, since they are both predicted by electron placement
The electron geometry will include the placement of lone pairs, whereas the molecular geometry ignores lone pairs and only considers the placement of atoms in its shape.
CRB What is the name of the number of atoms that will bond to the central atoms, and is often used when describing lattices?
(A) Bond number
(B) Bond order
(C) Coordination number
(D) Coordination order
(C) Coordination number
The coordination number describes the number of atoms that will bond to the central atoms, and is often used when describing lattices.
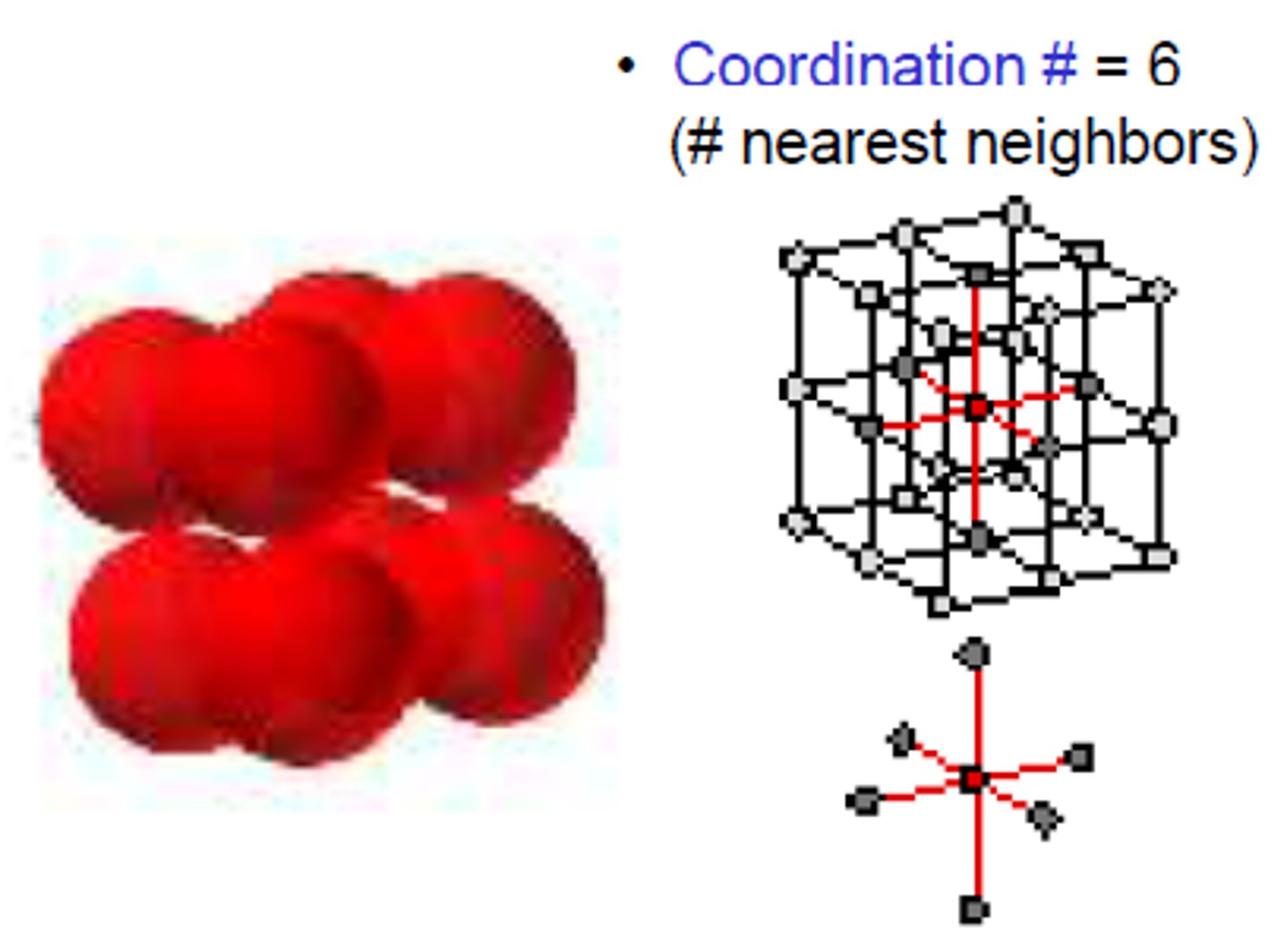
What is the purpose of VSEPR?
To help represent and predict the shape of a molecule.
CRB True or false? In VSEPR theory, a double bond is treated exactly the same as a single bond when determining electron and molecular shapes.
True. In VSEPR theory, a double bond is treated exactly the same as a single bond when determining electron and molecular shapes.
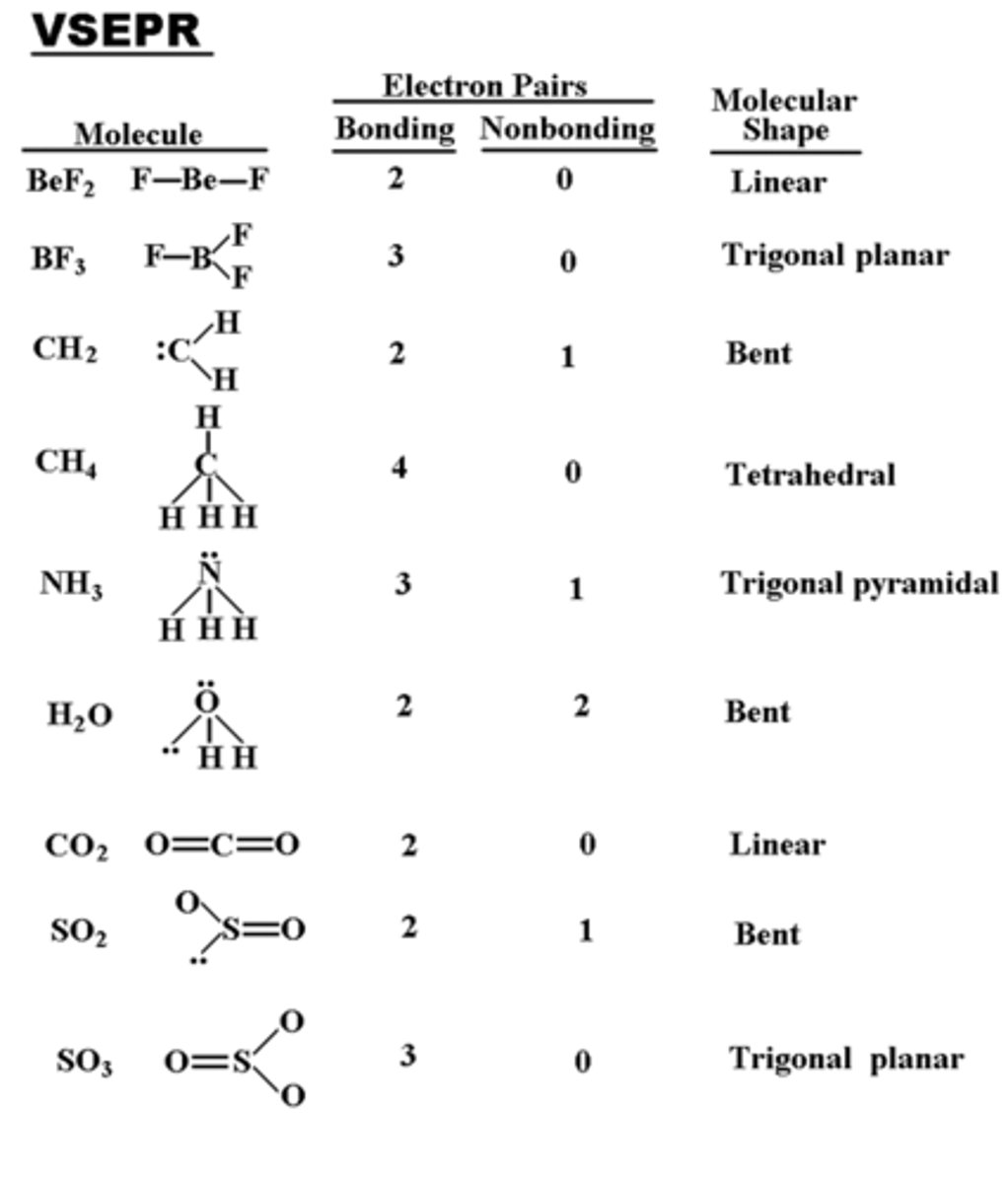
The first step of VSEPR is to draw the dot structure of the compound. What is the dot structure of BeCl2?
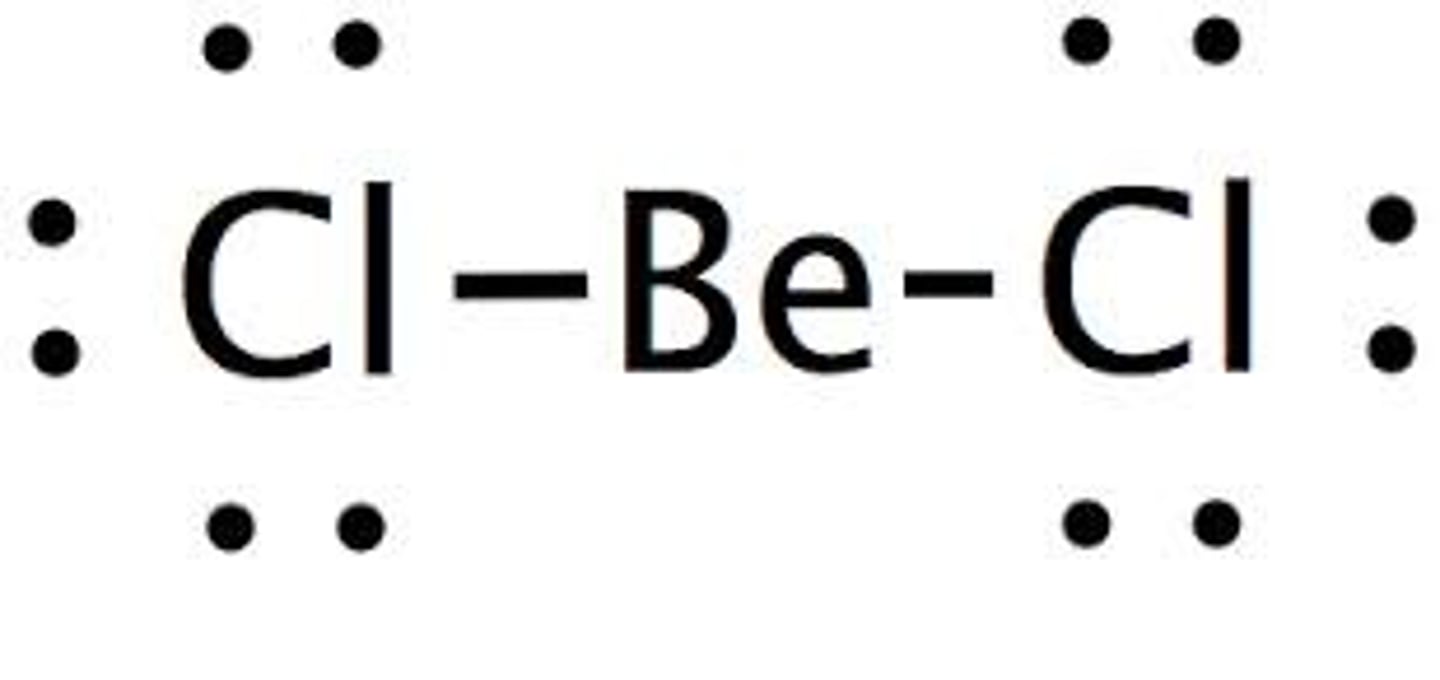
Why doesn't Chlorine share its lone pair of electrons with Berylium in BeCl2?
Because Berylium has a neutral formal charge with only one bond to each Chlorine. it would be less stable if it received more electrons from Chlorine.
What is the difference between electron geometry and molecular geometry?
Electron geometry is the shape of the electron clouds about the central atom.
Molecular geometry is the shape of the atoms about the central atom.
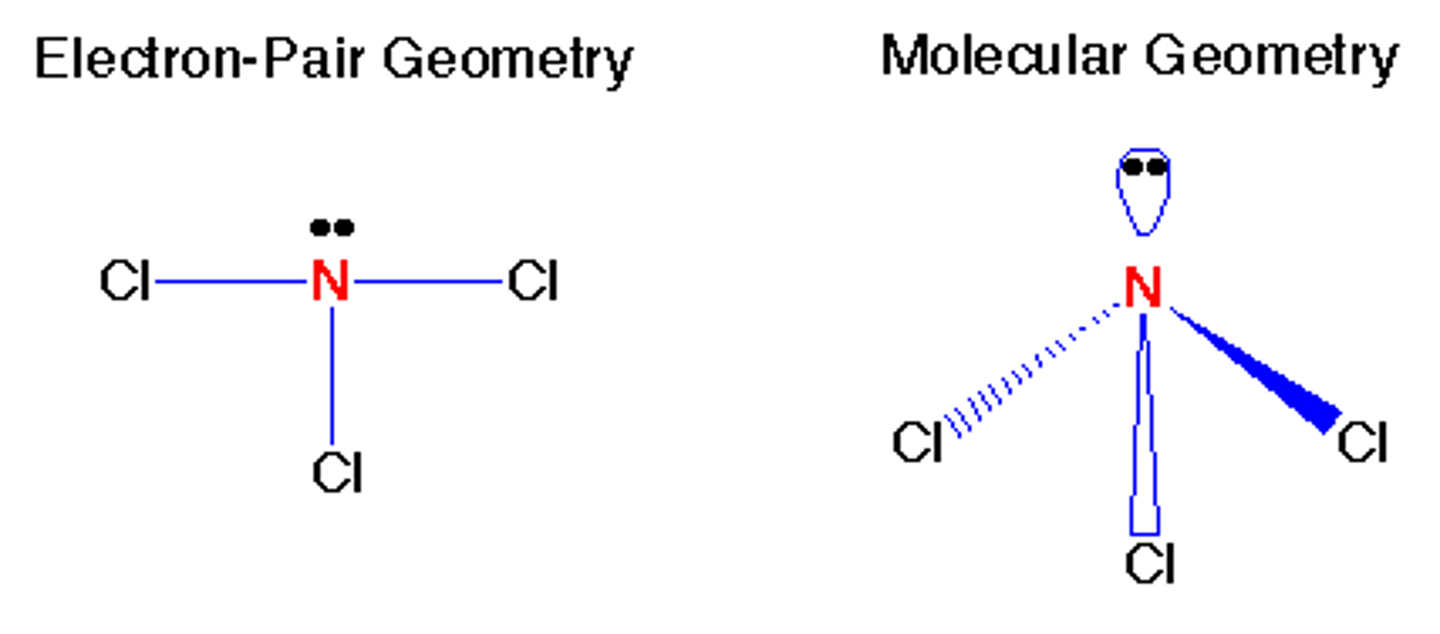
The next step in VSEPR is to count the number of "electron clouds" around the central atom. How many "electron clouds" are surrounding the central atom of BeCl2?
2 electron clouds.
What is the electron geometry of BeCl2? Molecular geometry?
Electron geometry of BeCl2 is linear.
Molecular geometry of BeCl2 is linear.
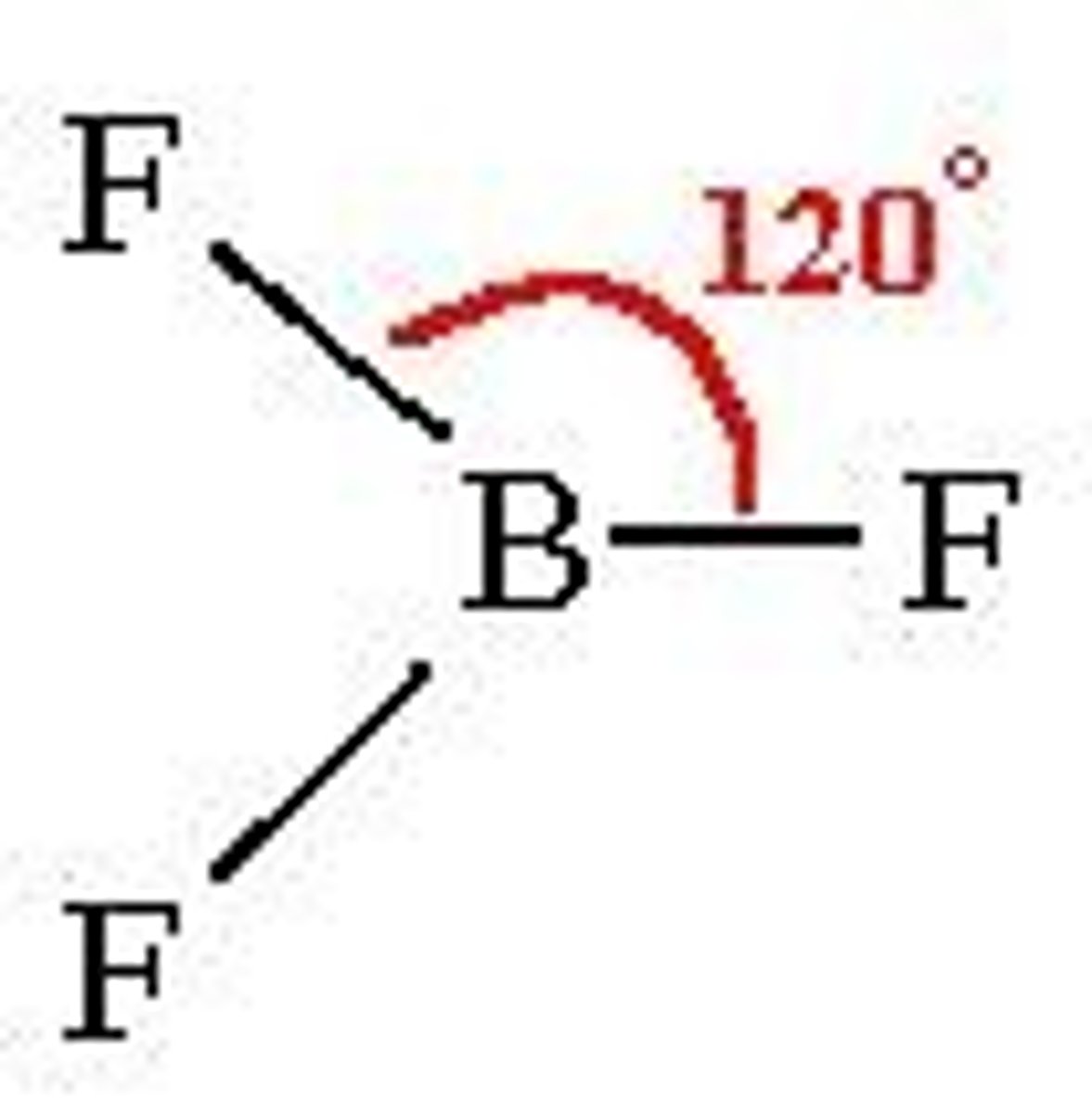
What is the electron geometry of BF3? Molecular geometry?
Electron geometry of BF3 is trigonal planar.
Molecular geometry of BF3 is trigonal planar.
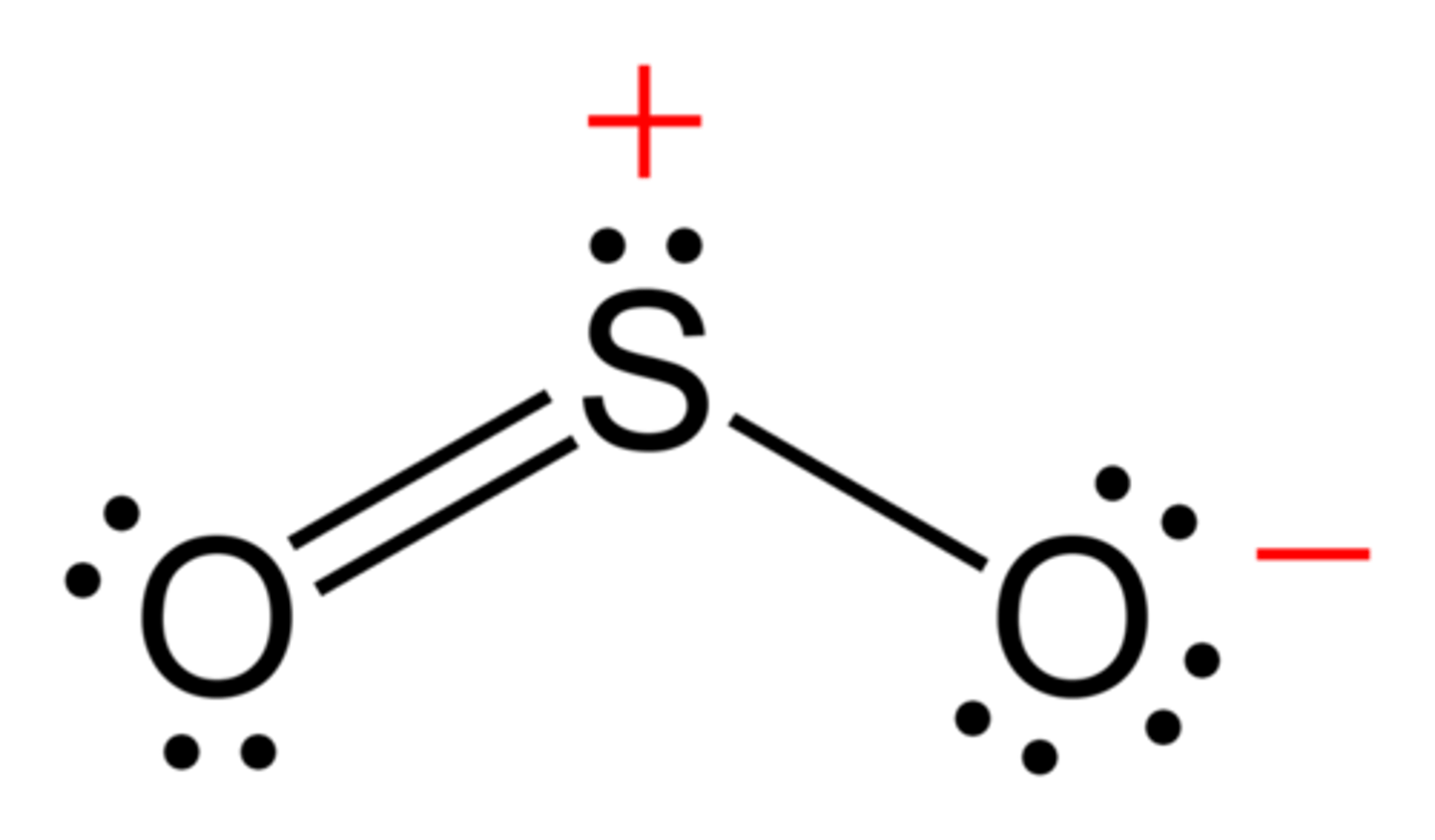
What is the electron geometry of Sulfur Dioxide? Molecular geometry?
Electron geometry of Sulfur Dioxide is trigonal planar.
Molecular geometry of Sulfur Dioxide is bent/angular.
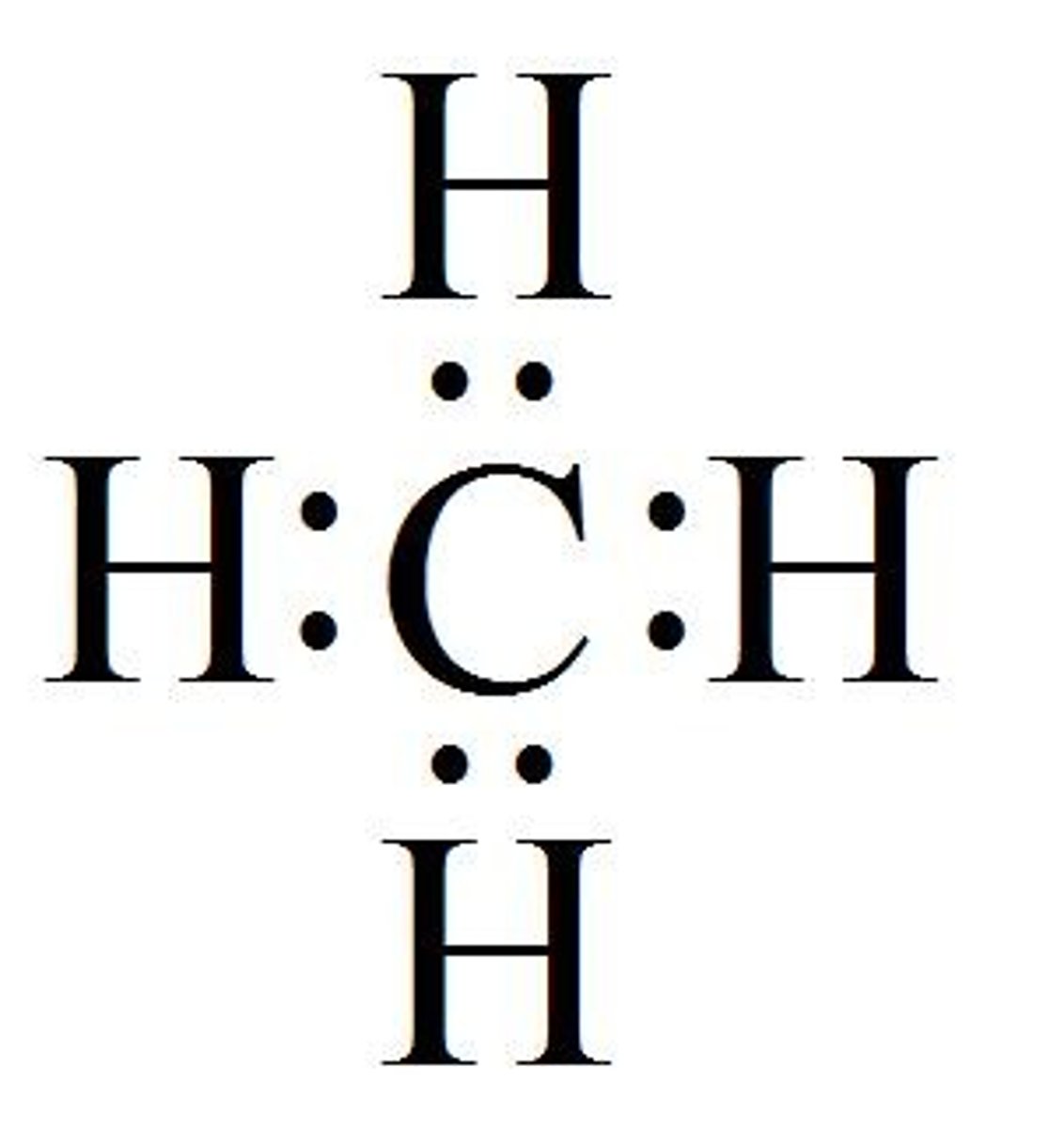
What is the electron geometry of CH4? Molecular geometry?
Electron geometry of CH4 is tetrahedral.
Molecular geometry of CH4 is tetrahedral.
CRB For a tetrahedral compound, which of the following is the ideal bond angle (in degrees)?
(A) 90
(B) 104.5
(C) 109.5
(D) 120
(C) 109.5
For a tetrahedral compound, the ideal bond angle is 109.5 degrees.
What is the electron geometry of NH3? Molecular geometry?
Electron geometry of NH3 is tetrahedral.
Molecular geometry of NH3 is trigonal pyramidal.
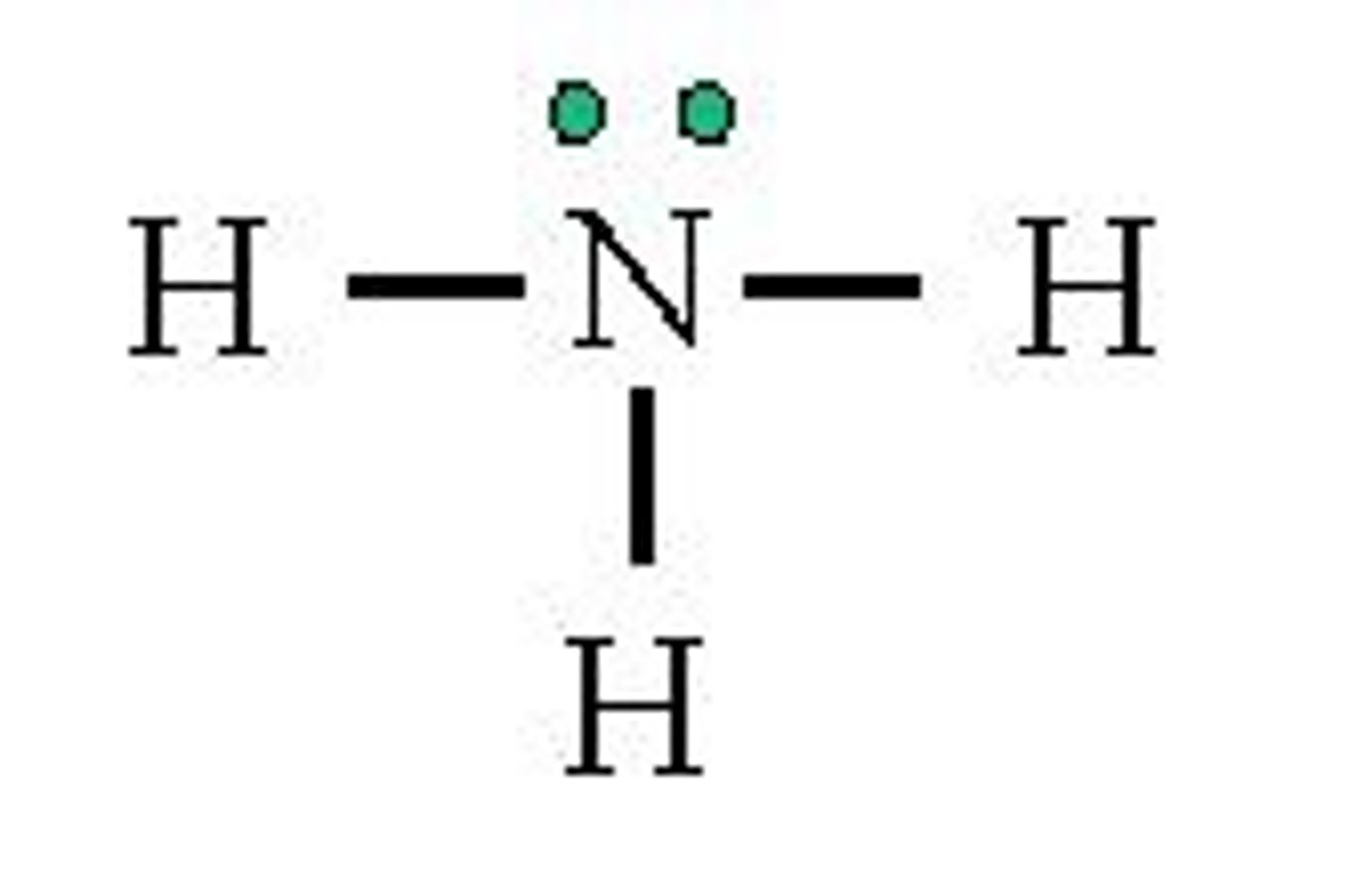
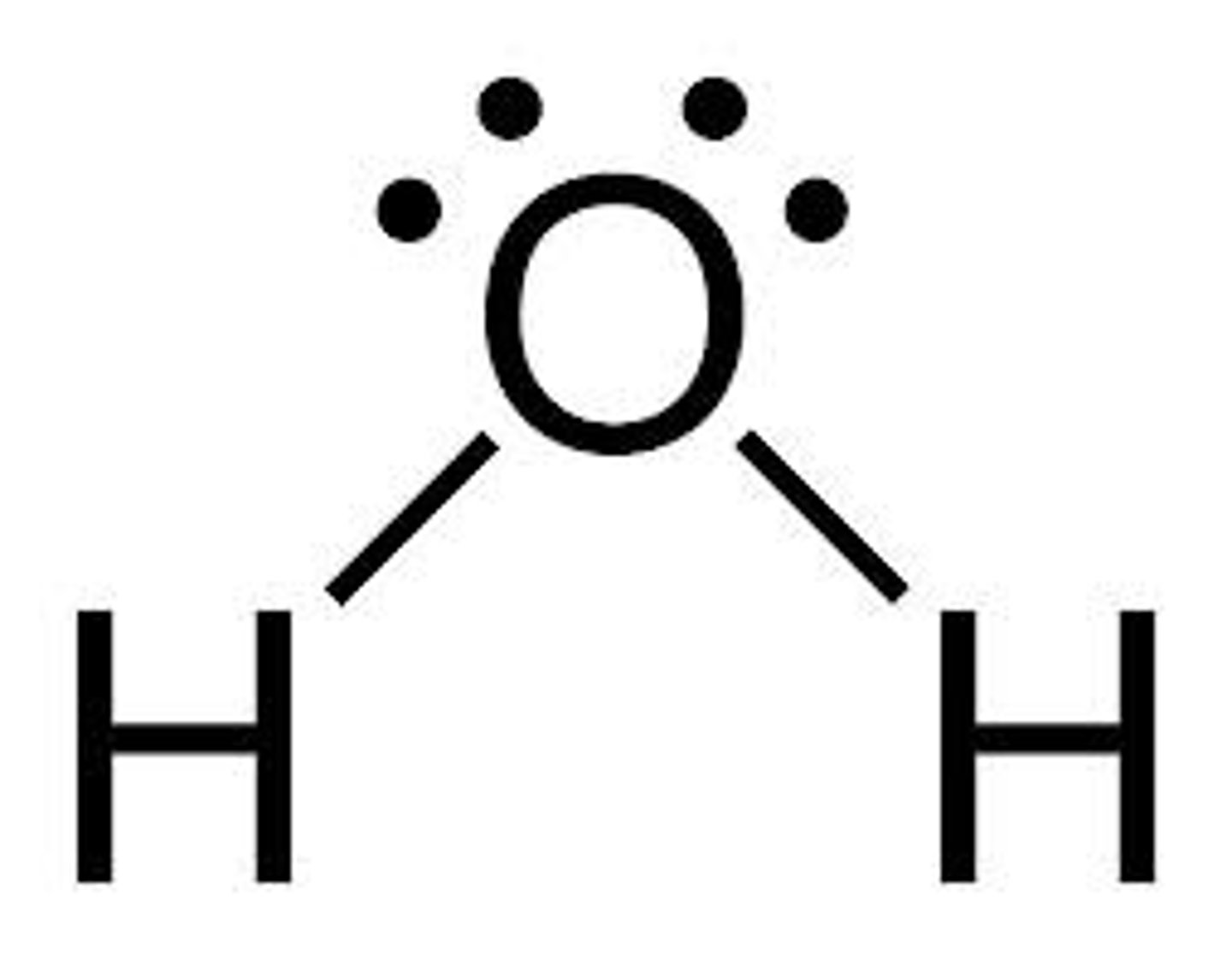
What is the electron geometry of H2O? Molecular geometry?
Electron geometry of H2O is tetrahedral.
Molecular geometry of H2O is bent.
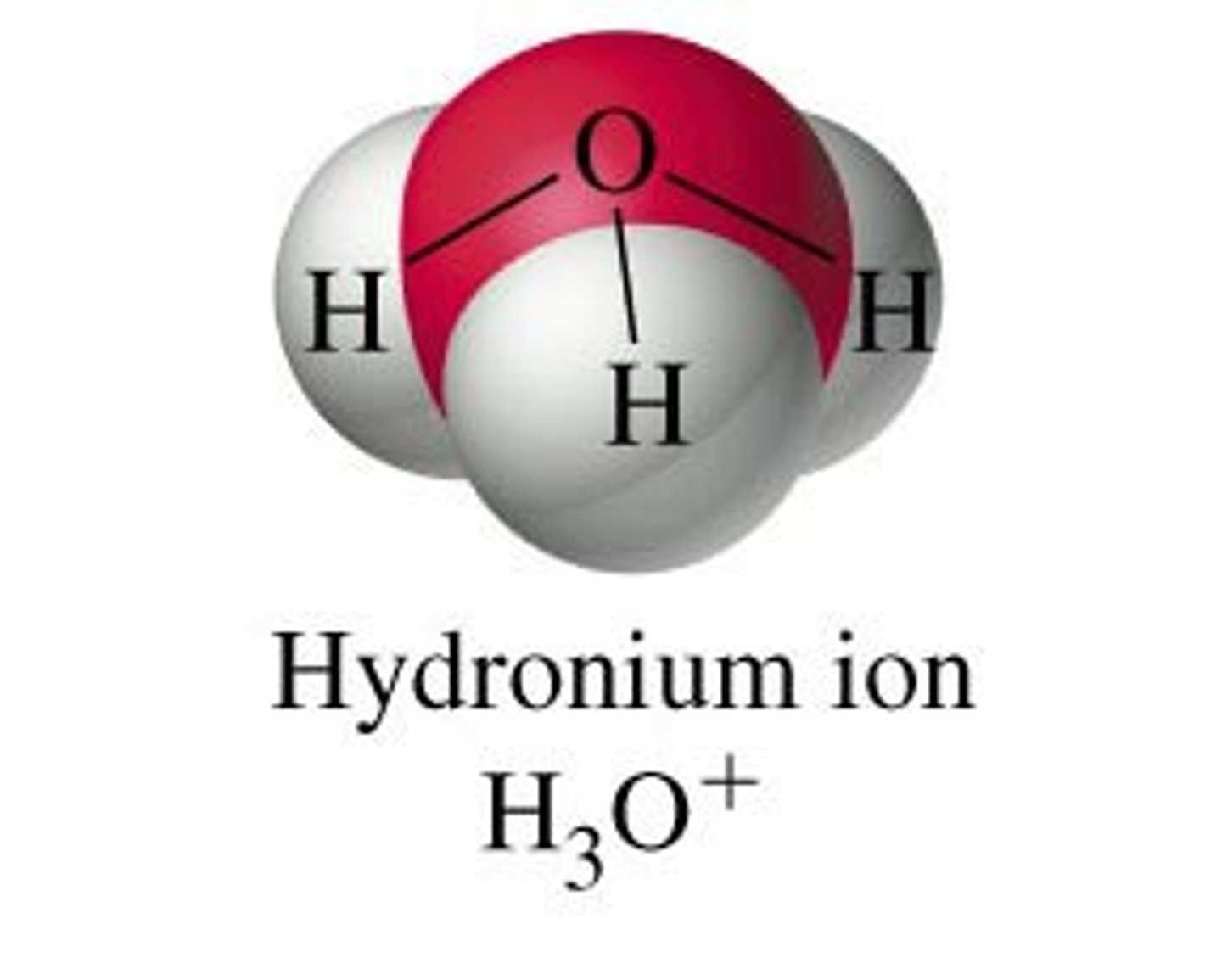
What is the electron geometry of H3O+? Molecular geometry?
Electron geometry of H3O+ is tetrahedral.
Molecular geometry of H3O+ is trigonal pyramidal.
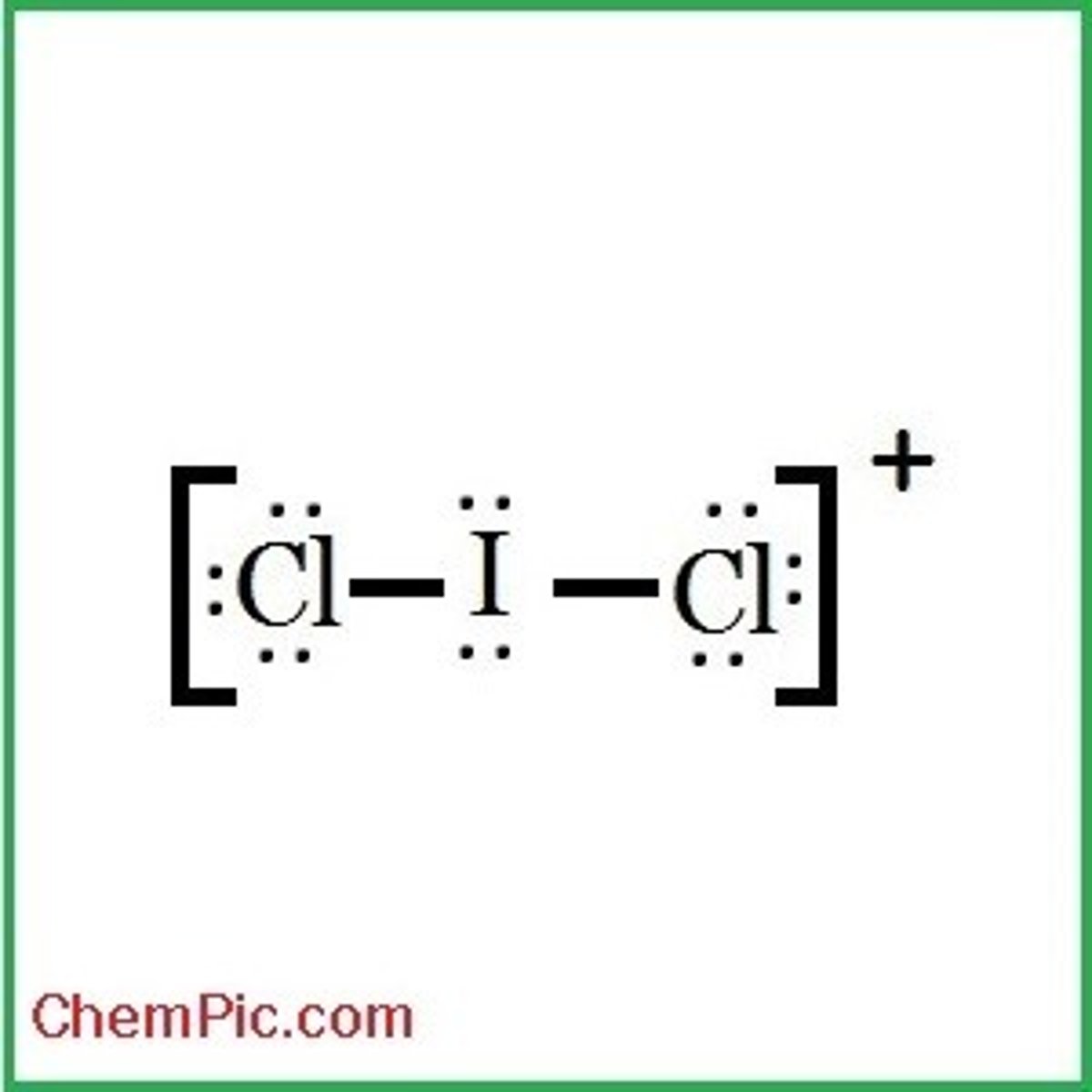
What is the Electron Geometry of ICl2+? Molecular Geometry?
Electron Geometry of ICl2+ is Tetrahedral.
Molecular Geometry of ICl2+ is Bent.
What is the electron geometry of CO2? Molecular geometry?
Electron geometry of CO2 is linear.
Molecular geometry of CO2 is linear.
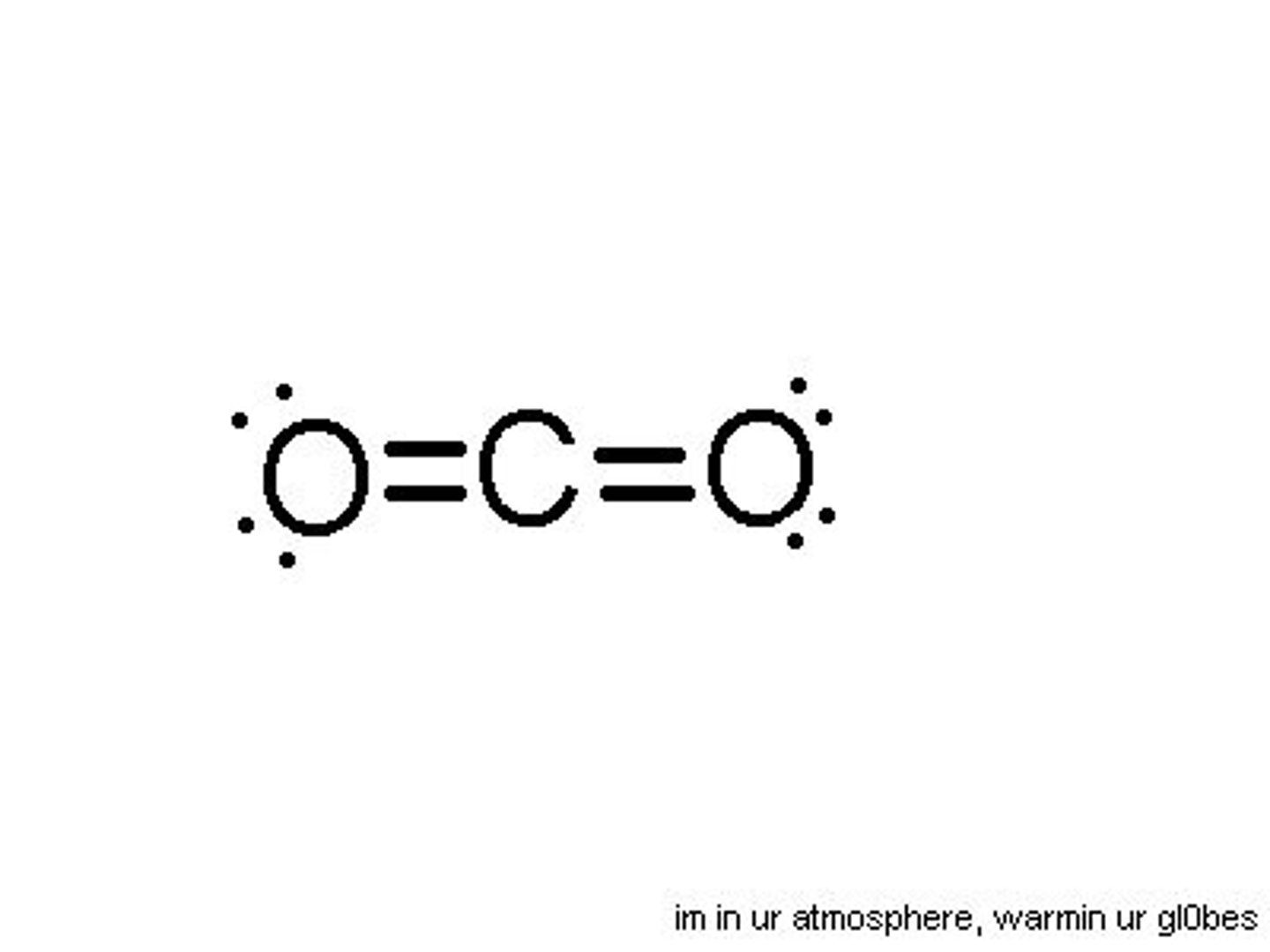
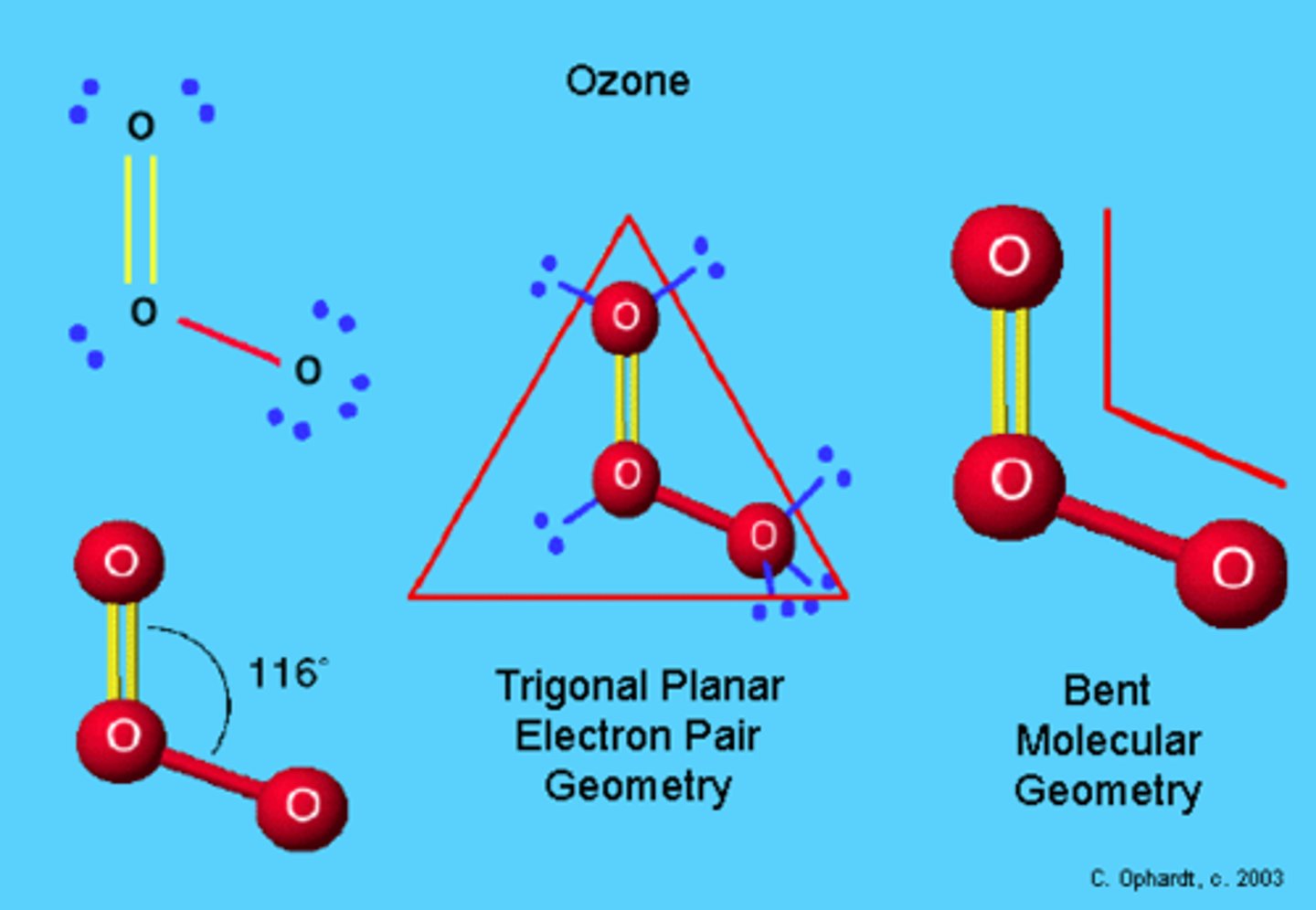
What is the electron geometry of O3? Molecular geometry?
Electron geometry of O3 is trigonal planar.
Molecular geometry of O3 is bent/angular.
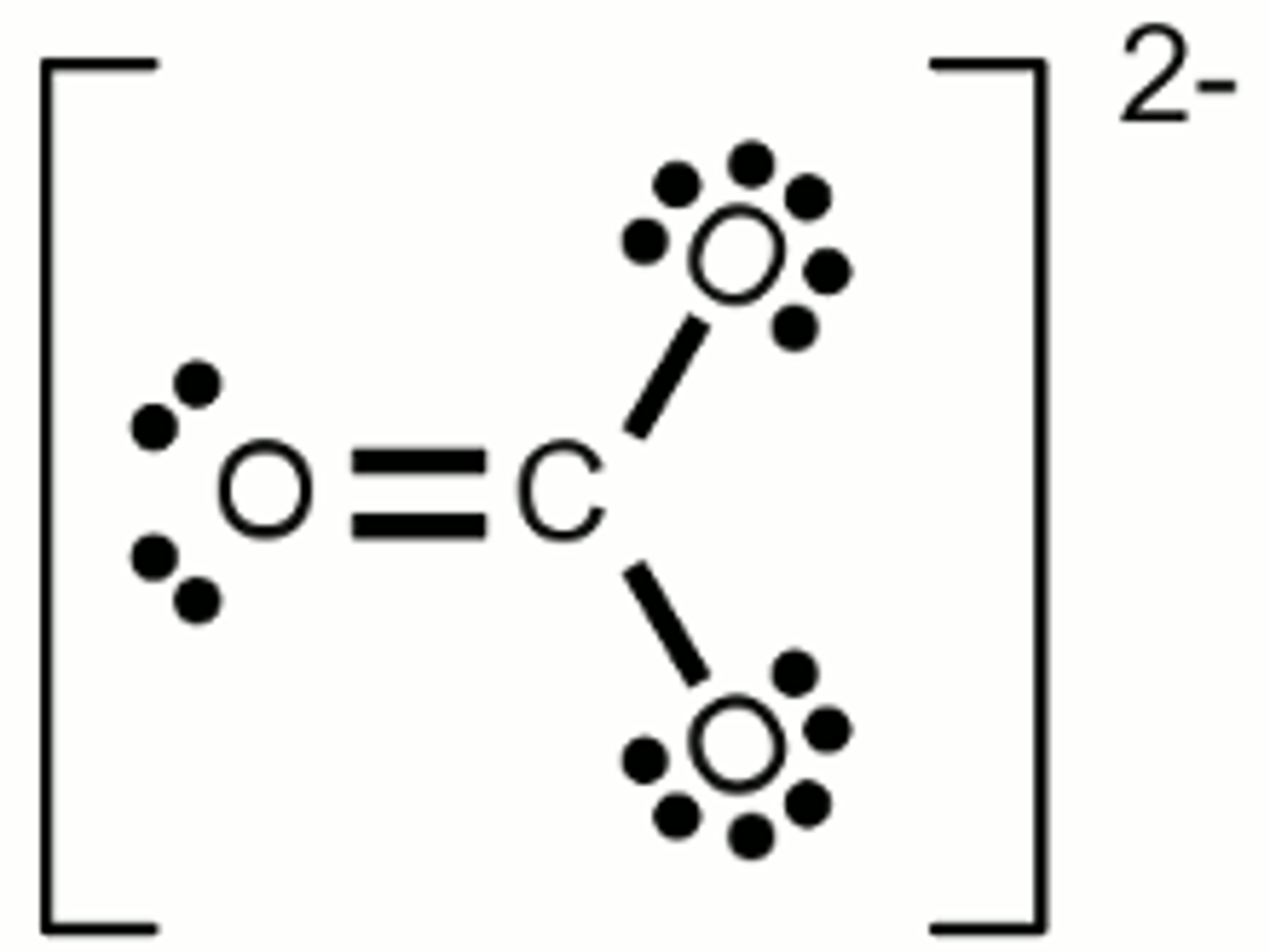
What is the electron geometry of Carbonate? Molecular geometry?
Electron geometry of Carbonate is trigonal planar.
Molecular geometry of Carbonate is trigonal planar.
What is the electron geometry of Sulfate? Molecular geometry?
Electron geometry of Sulfate is tetrahedral.
Molecular geometry of Sulfate is tetrahedral.