Chemistry-Isotopes
1/8
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
9 Terms
strontium 90
used to determine the thickness of metal sheets

use of cobalt 60
radiotherapy- used to treat and cure cancer cells
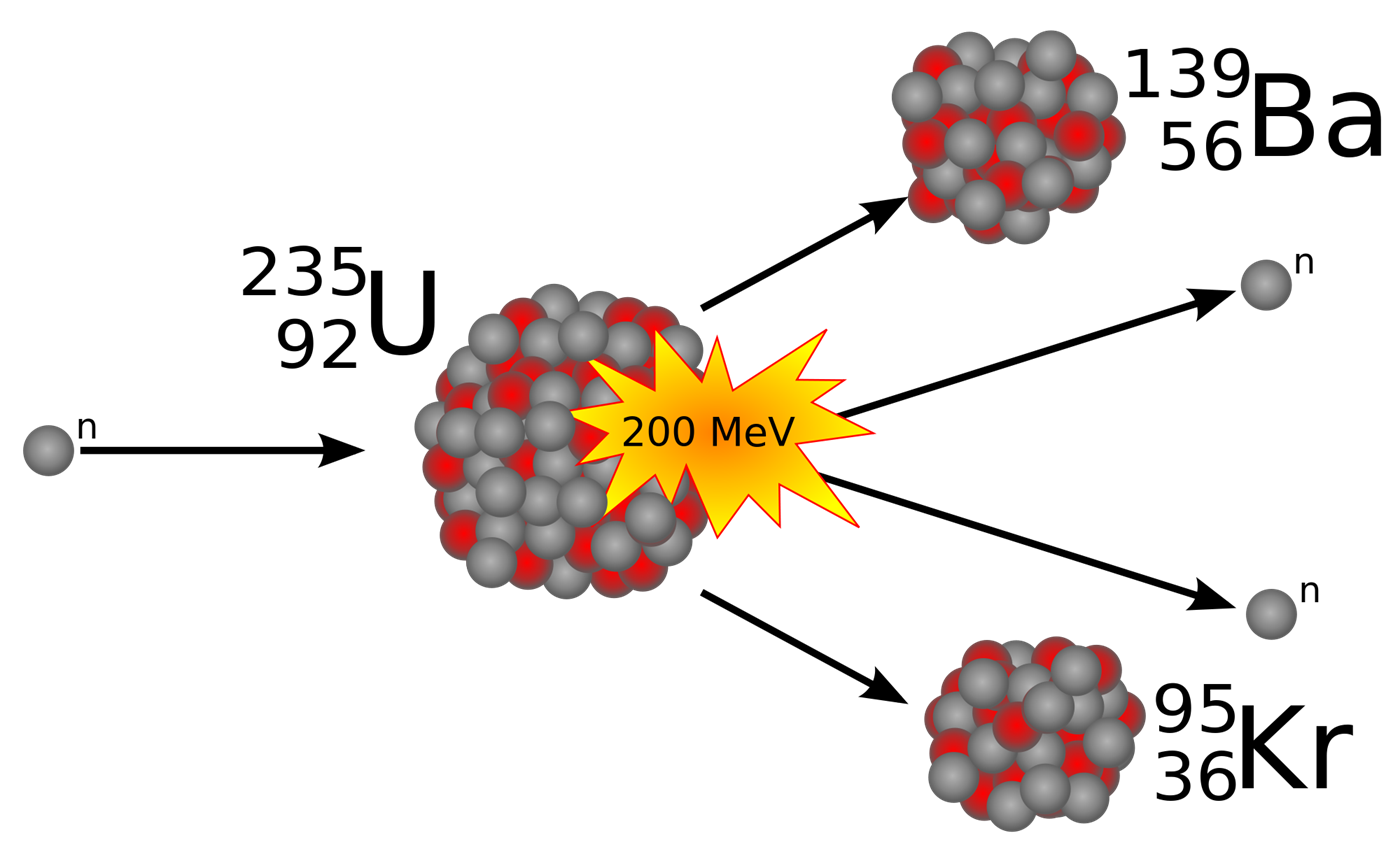
uranium 235
used in nuclear power generation
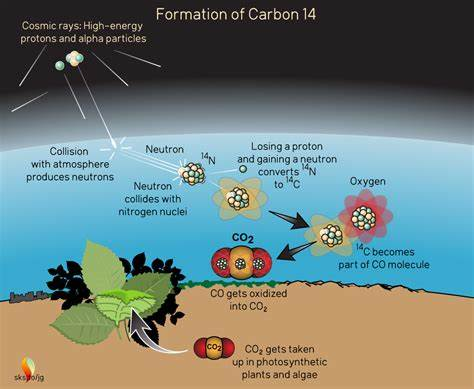
carbon 14
determine when an organism died up to 50 000 years
example isotopes of carbon
1. Carbon-12 (¹²C)
• Atomic Structure: 6 protons + 6 neutrons
• Carbon-13 (¹³C)
• Atomic Structure: 6 protons + 7 neutrons
• Carbon-14 (¹⁴C)
• Atomic Structure: 6 protons + 8 neutrons
radioactive decay
the process of emitting radioactive particles from unstable nuclei to make the nuclei more stable
radioactive isotopes
an isotope that releases / emits radioactive particles spontaneously to become more stable. WHY- due to unstable nuclei due to different number of protons and neutrons.
isotopes
atoms with same atomic number but different nucleon numbers