Aromatic chemistry
1/21
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
22 Terms
what is a benzene
sweet smelling organic compounf
formula for benzene
C6H6
Kekule’s Model
Alternating single and double carbon-carbon bonds
problems with Kekules model
Doesn’t undergo addition reaction like expected
the carbon - carbon bond lengths are identical
Enthalpy of hydrogenation is less exothermic than expected
how is the pi system made
instead of 3 pi bonds remaining pi orbitals overlap with both neighboring pi orbitals for a pi systerm
what dies pi sytem do to electrons
electrons are delocalized , same in any alternating single and double bond molecule
why is compund with delocalised electron more stable than ones that have localised bond
delocalised electrons spread out , deacrsing electron density
what is an aromatic compound
compound with benzen ring
why does benzen not undergo addition reaction like expected
lower electron density than aleknes
why does carbon length not identical in benzene ring
each p bond overlaps with neighboring instead of forming a pi bonds
why is enthalpy of hydrogination less
benzene delocalized electron , more stable
naming aromatic compounds priority list
Carboxylic acid
Nitriles
Aldehydes
ketones
alcohols
alkene
benzene
halogenoalkanes
alkane
nitro
below benzene - prefix benzene
above benzene - prefix phenyl
Friedel- craft acylation reaction
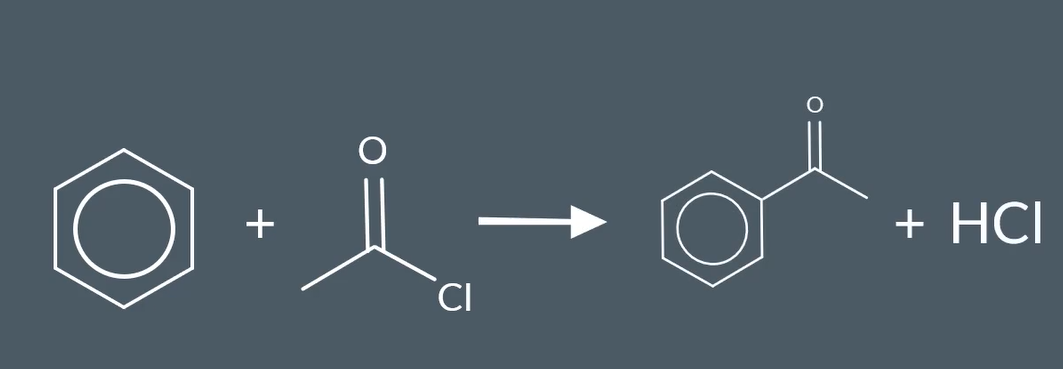
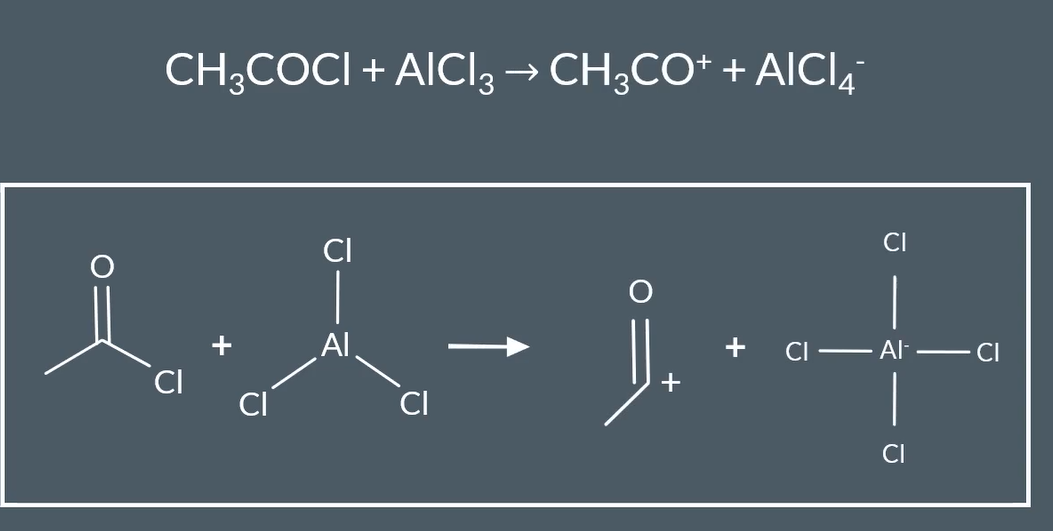
why is catalyst AlCl3 needed for Friedel - craft acylation
makes the electrophile stronger
reaction in which AlCl3 to make electrophile stronger
word to describe the AlCl3 in fridel-craft othr than catalyst
halogen carrier
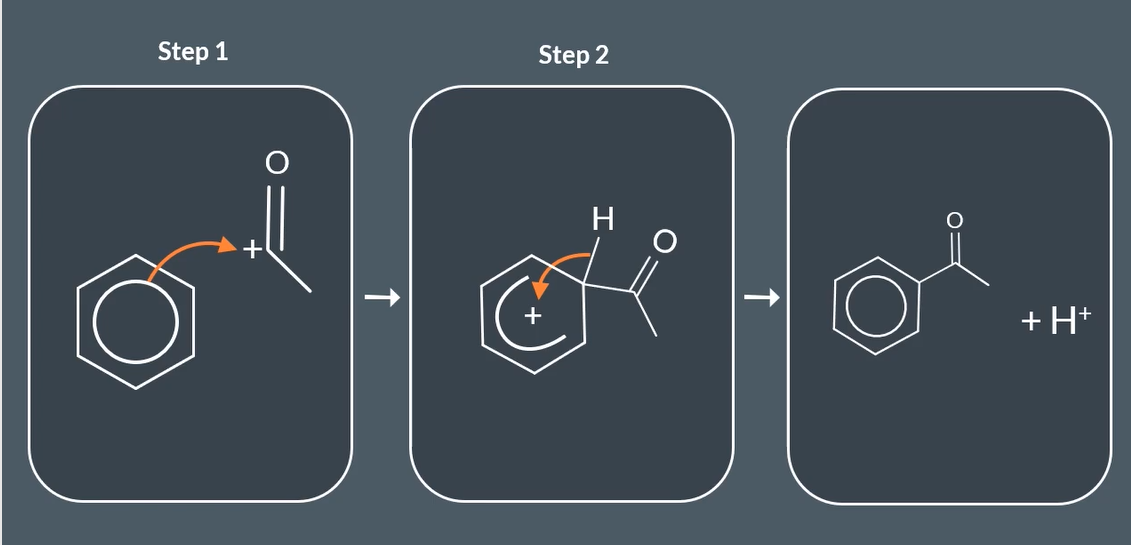
step mechanism for fridel- craft
equation for regeneration with AlCl3

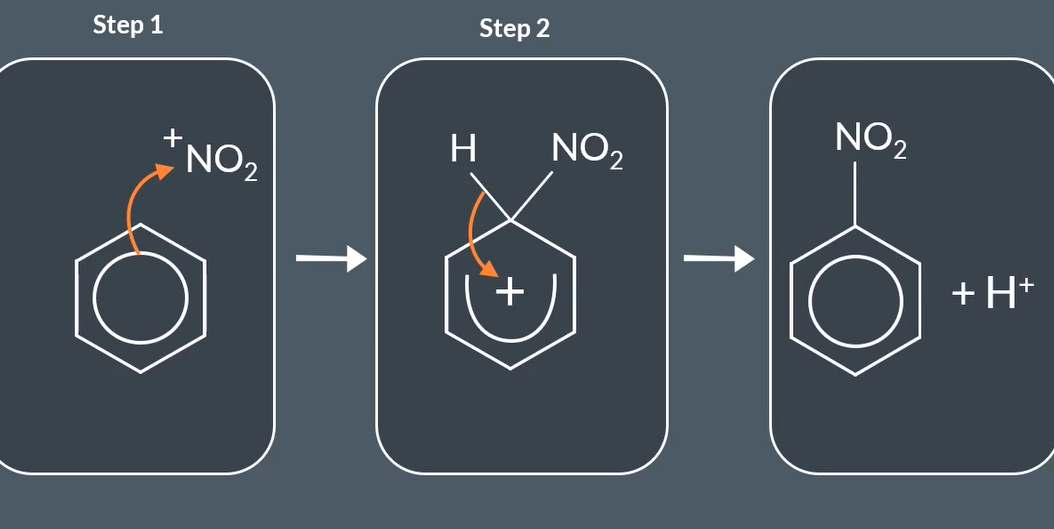
equation for nitration of benzene
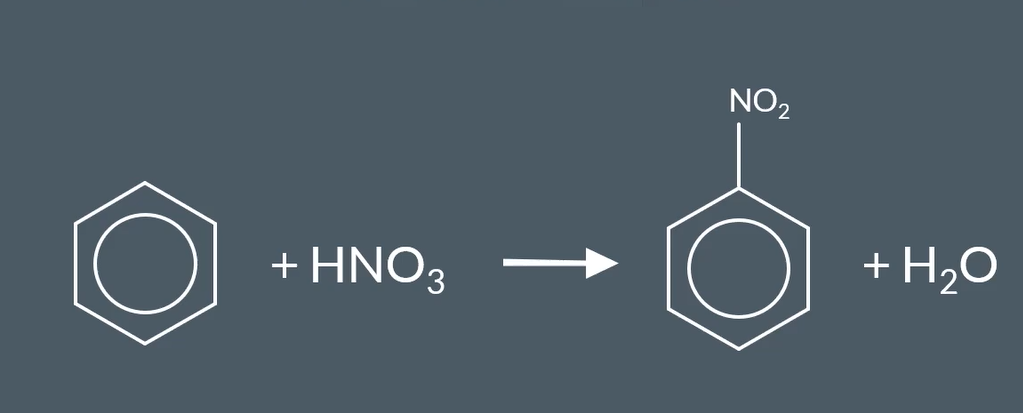
what catalyst used in nitration of benzene and what for
conc H2so4
TO MAKE STRONGER CATALYST
equation for H2SO4 to make electrophile stronger and electrophile used
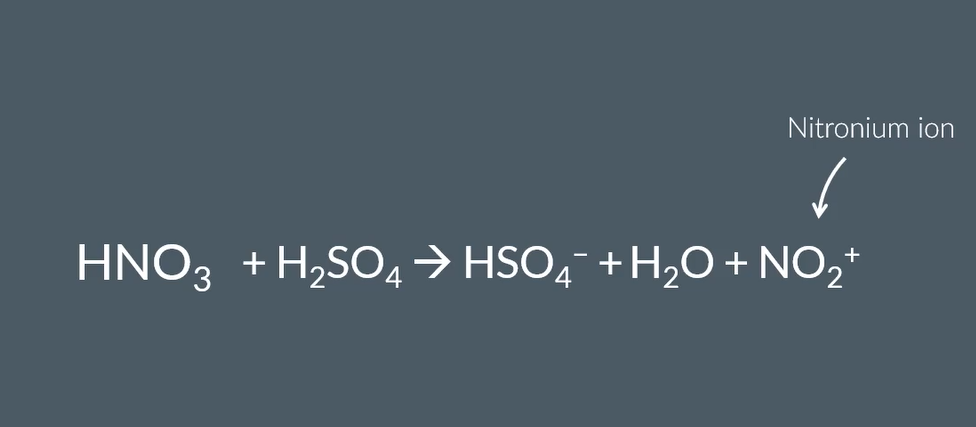
step mechanism for nitration of benzene