Immunology - Lecture 13 - Lymphocyte Receptor Signaling
1/41
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
42 Terms
What is the main function of transmembrane receptors?
They convert extracellular signals into intracellular biochemical events.
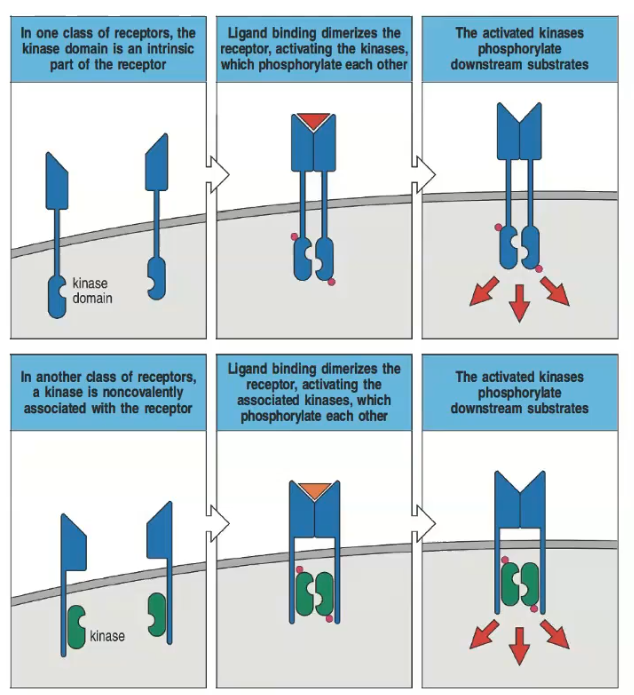
What are the two main types of transduction receptors?
1) Receptors with an intrinsic intracellular kinase domain.
2) Receptors with a non-covalent attachment of a kinase.
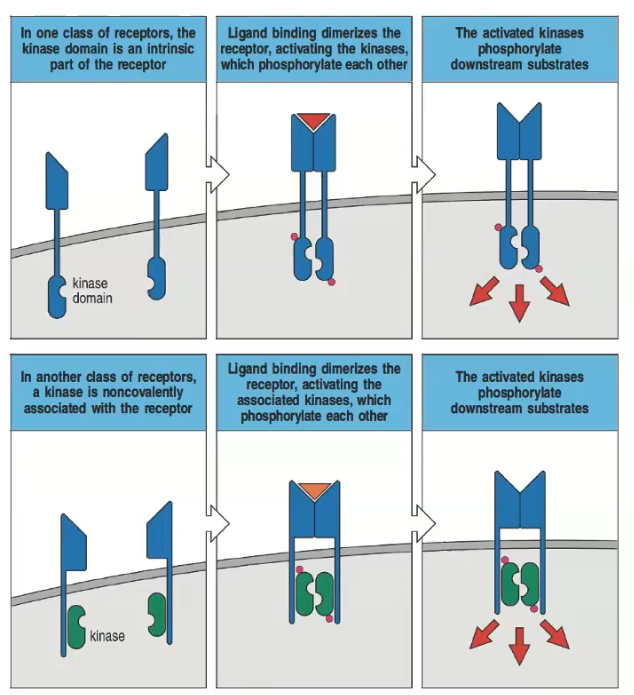
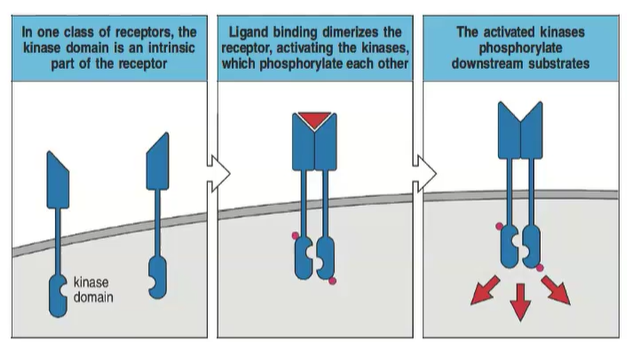
How do receptors with an intrinsic kinase domain signal upon ligand binding?
Ligand binding dimerizes the receptor, activating the kinases which phosphorylate each other and downstream substrates.
Describe the process in receptors that have a non-covalently associated kinase.
Ligand binding dimerizes the receptor, activating the associated kinases, which then phosphorylate each other and downstream substrates.
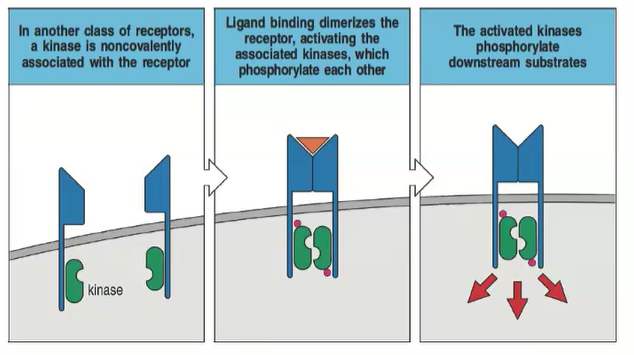
What role does dimerization play in transmembrane receptor signaling?
Dimerization upon ligand binding activates kinase phosphorylation, initiating signal transduction.
What happens after transmembrane receptors are activated?
The activated receptors phosphorylate downstream substrates, transmitting the signal through various adaptor proteins, kinases, or phosphatases.
In transmembrane receptor signaling, what can occur after ligand binding to membrane receptors?
The receptors dimerize and may either autophosphorylate or recruit kinases to propagate the signal.
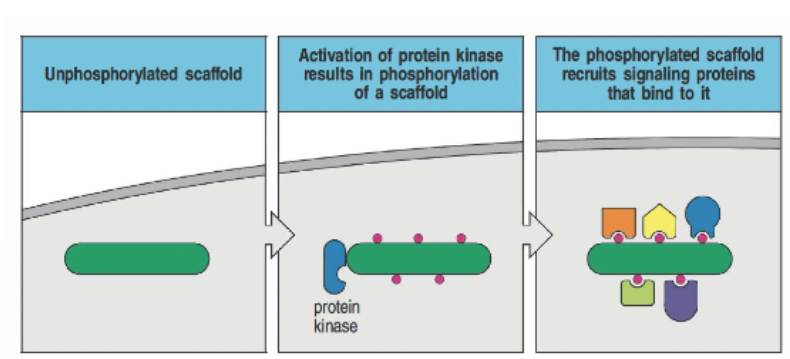
How does phosphorylation of scaffolds and adaptor proteins lead to intracellular signaling?
An unphosphorylated scaffold undergoes phosphorylation by a protein kinase, which allows it to recruit additional signaling proteins. These proteins dock onto the scaffold via specific domains, such as SH2 and SH3, which recognize phosphate groups or proline-rich sequences. This assembly facilitates the formation of signaling complexes within the cell membrane, enabling further downstream signaling.
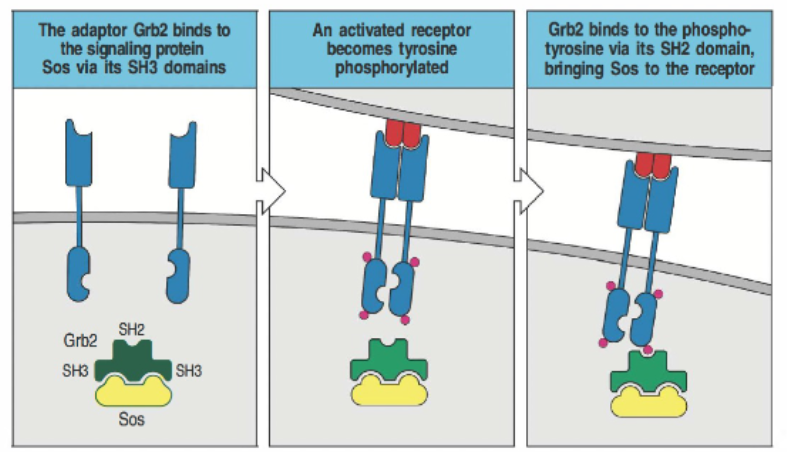
Describe the specific role of the adaptor protein Grb2 in scaffold-mediated signaling.
Grb2 binds to the signaling protein SOS via its SH3 domain, which recognizes proline-rich sequences. When the receptor is activated and becomes tyrosine-phosphorylated, Grb2’s SH2 domain binds to the phosphotyrosine on the receptor. This interaction brings SOS to the receptor, allowing it to participate in further signaling processes.
How is intracellular signal propagation mediated, and what role do scaffolds and adaptors play?
Intracellular signaling is driven by multiprotein complexes formed through scaffolds and adaptors.
Scaffolds have phosphorylated tyrosine sites that recruit proteins.
After TCR or BCR activation, signals are integrated through ligands like phosphotyrosine, proline, phosphoinositides, protein C termini, membrane lipids, and polyubiquitin.
What are Src-homology 2 (SH2) domains, and what is their role in cell signaling?
Src-homology 2 (SH2) domains are conserved protein domains in TCR and BCR signaling.
They consist of an antiparallel beta sheet and two alpha helices, around 100 amino acids.
SH2 domains recognize phosphorylated tyrosines (pY) in a pYXXZ sequence, where X is any amino acid and Z is specific.
They allow proteins, including scaffolds, to bind phosphorylated tyrosines, aiding in signaling.
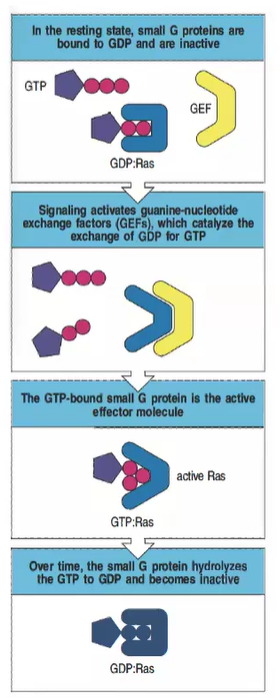
What is the inactive state of small G proteins, and how is it maintained?
In the resting state, small G proteins like Ras are bound to GDP and remain inactive.
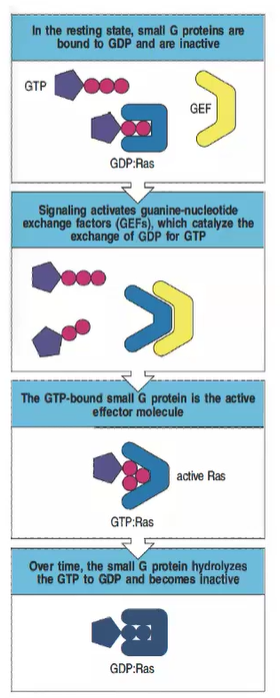
How are small G proteins activated in intracellular signaling?
Signaling activates guanine-nucleotide exchange factors (GEFs), which catalyze the exchange of GDP for GTP, activating the small G protein.
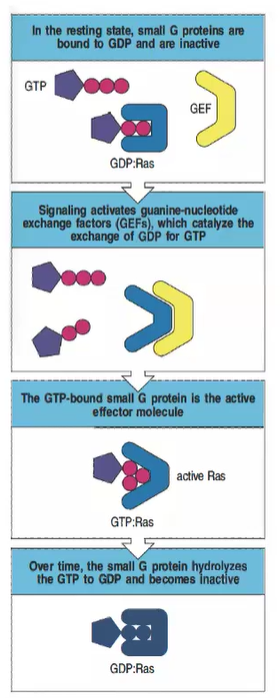
What is the active form of Ras, and what does it do?
The GTP-bound form of Ras is active and can act as an effector molecule to propagate downstream signaling.
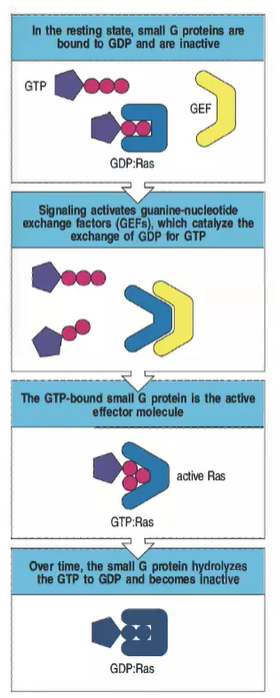
What role do GTPase-activating proteins (GAPs) play in regulating small G proteins?
GAPs promote the hydrolysis of GTP to GDP, returning the small G protein to its inactive state.
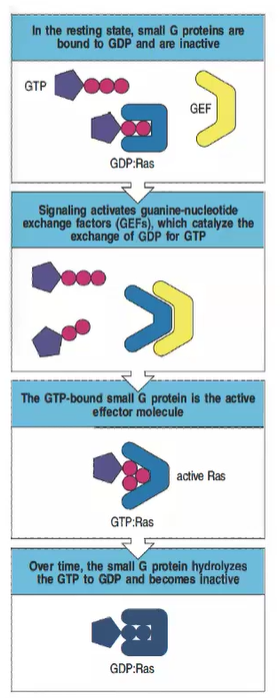
Why are small GTPases, like Ras, important in cell signaling, and how are they regulated?
Small GTPases regulate various cellular processes, including proliferation and actin changes. They are regulated by GEFs (which activate them by binding GTP) and GAPs (which inactivate them by promoting GTP hydrolysis).
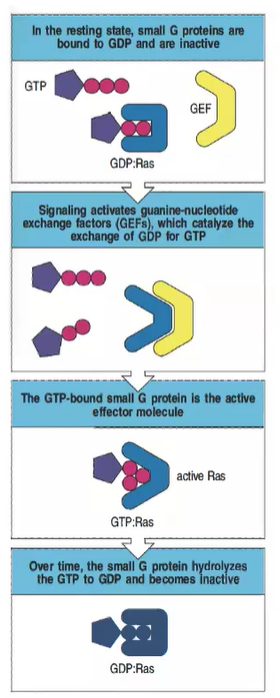
How can mutations in Ras contribute to cancer?
Certain Ras mutations prevent GAP-mediated GTP hydrolysis, locking Ras in the active GTP-bound state, leading to uncontrolled signaling that can contribute to cancer.
How does the modification of PIP2 contribute to intracellular signaling?
PIP2 is phosphorylated by PI3 kinase to form PIP3.
PIP3, a membrane-bound lipid, is recognized by kinases like Akt and Itk via their PH domains.
This recognition enables kinases to engage in downstream signaling and regulate cellular processes.
How does dephosphorylation regulate cell-surface receptor signaling?
Dephosphorylation removes phosphate groups from phosphorylated substrates, dampening the signaling activated by kinases. This reversible process helps regulate and control the duration of signaling.
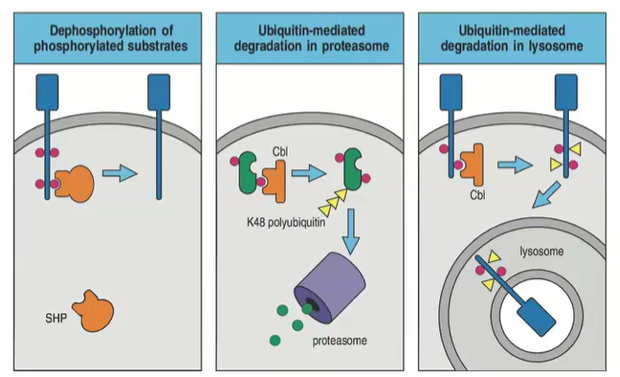
What role does ubiquitination play in the regulation of cell-surface receptor signaling?
Ubiquitination targets proteins for degradation, shutting off signaling.
Polyubiquitinated cytosolic proteins go to the proteasome.
Polyubiquitinated membrane-bound proteins are sent to the lysosome.
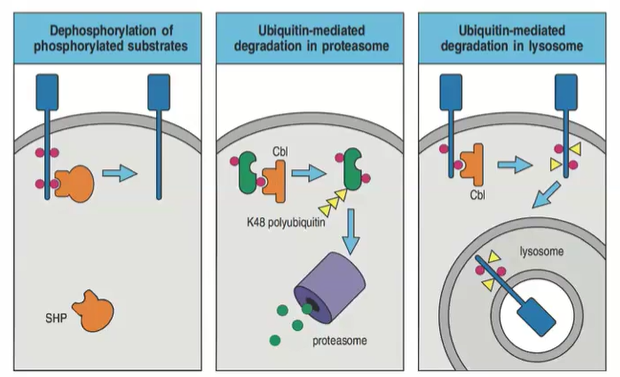
How do proteasome and lysosome pathways differ in degrading ubiquitinated proteins?
Proteasomes degrade polyubiquitinated cytosolic proteins; lysosomes degrade polyubiquitinated membrane proteins, permanently stopping signaling.
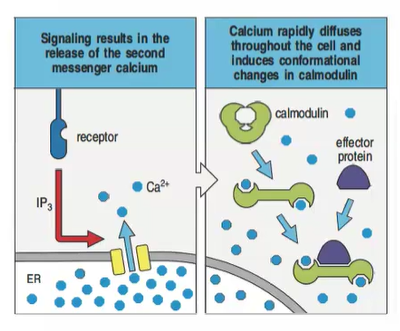
How does calcium act as a second messenger in intracellular signaling?
Phospholipase C-γ, activated by cell-surface receptors, hydrolyzes PIP2 into IP3 and DAG.
IP3 triggers Ca²⁺ release from the endoplasmic reticulum.
Ca²⁺ binds to calmodulin, forming a complex that activates effector proteins.
This complex regulates phosphorylation, dephosphorylation, and activates transcription factors, influencing gene expression.
4o
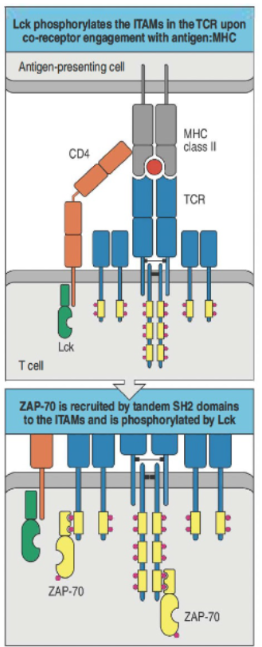
How is T cell receptor (TCR) signaling activated and amplified?
TCR signaling relies on coreceptors (CD3, CD4, CD8, and CD28) to form a multi-subunit complex.
Binding of TCR to an MHC-peptide complex on an APC causes phosphorylation of ITAMs in the CD3 complex.
Each ITAM has two tyrosine residues that, once phosphorylated, recruit kinases like ZAP70.
ZAP70 binds via its SH2 domain, amplifying the TCR signal and promoting T cell activation.
How is the phosphorylation of the T cell receptor (TCR) regulated?
Lck (bound to CD4/CD8) phosphorylates ITAMs in the TCR complex.
Phosphorylated ITAMs recruit ZAP-70 via its SH2 domains, initiating signaling.
CD45 regulates signal strength by dephosphorylating as needed.
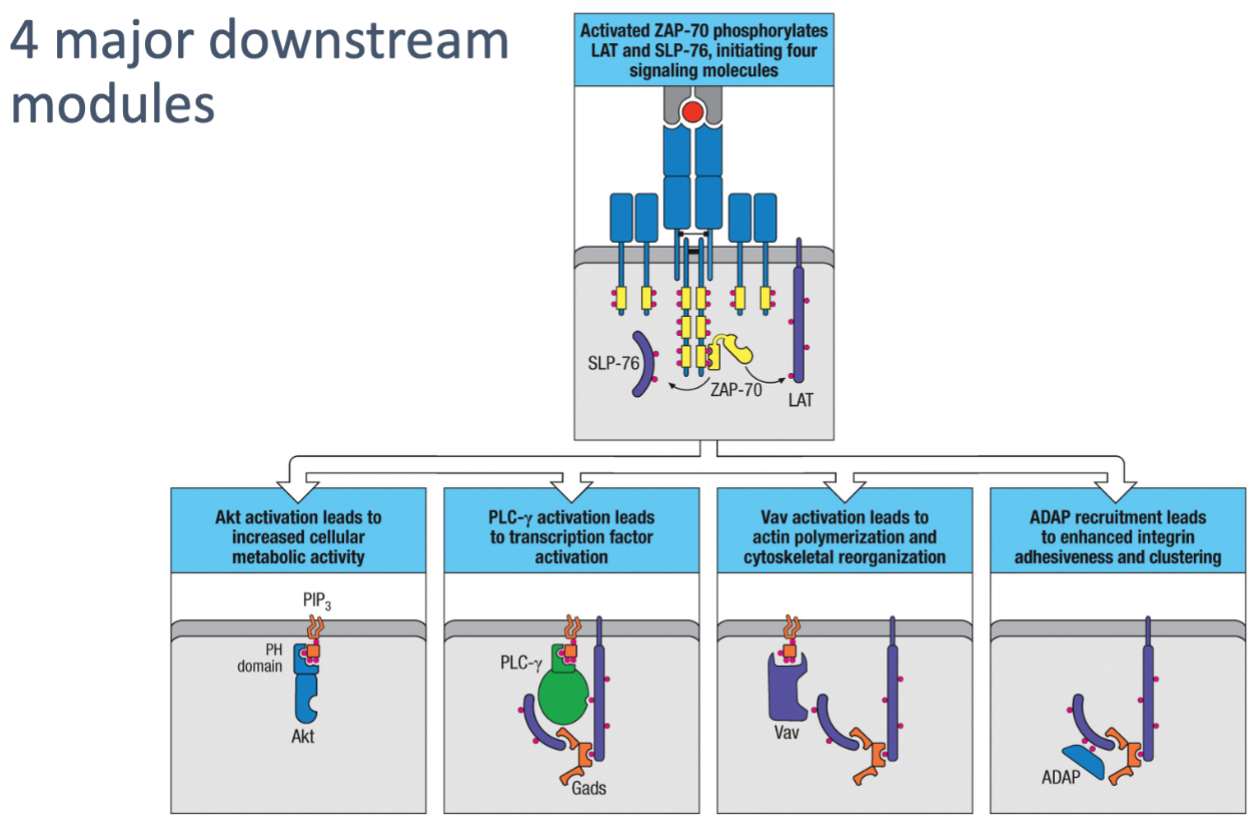
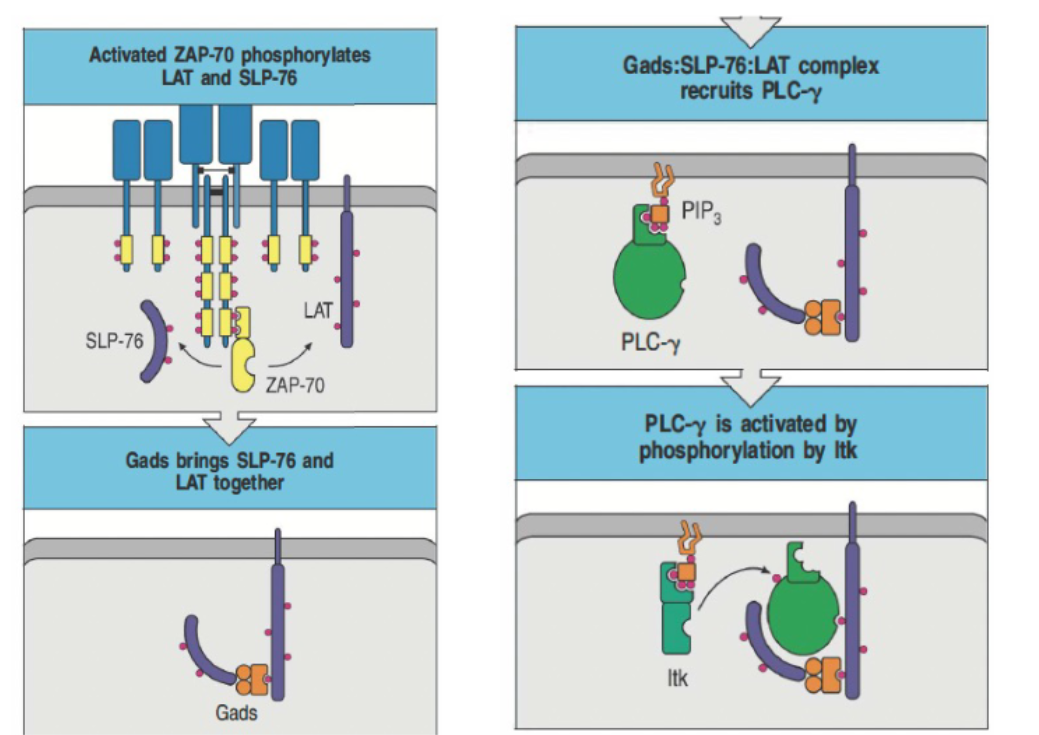
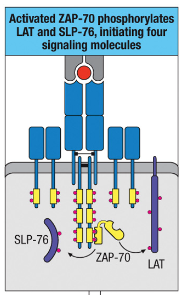
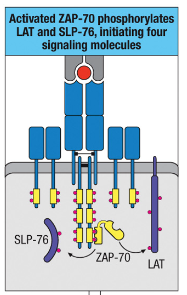
How does ZAP-70 contribute to the formation of a signaling complex involving LAT and SLP-76?
ZAP-70 phosphorylates LAT and SLP-76, enabling complex formation with Gads.
Gads complex recruits and activates PLC-γ via Itk phosphorylation.
This complex is essential for intracellular lipid signaling.
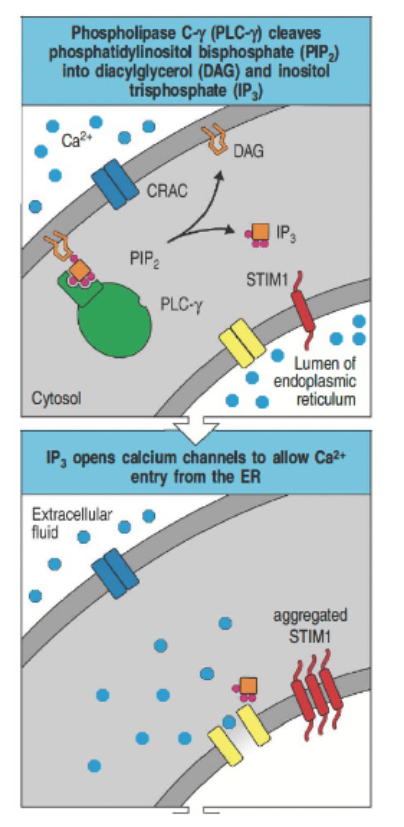
What role does phospholipase C (PLC) play in calcium signaling?
PLC cleaves PIP₂ into DAG and IP₃.
IP₃ binds to ER receptors, releasing Ca²⁺ into the cytosol.
Ca²⁺ interacts with calmodulin, activating calcineurin.
Calcineurin dephosphorylates NFAT, allowing it to enter the nucleus and regulate gene expression.
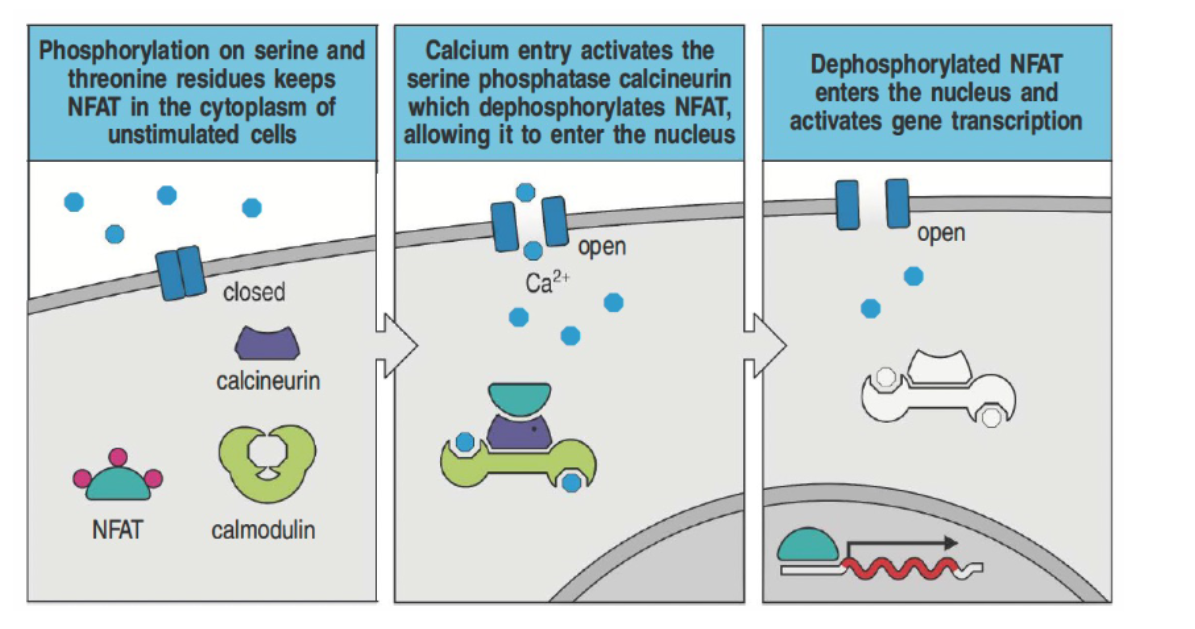
How does calcium signaling lead to gene activation in T cells through NFAT?
In resting cells, NFAT is kept in the cytoplasm by phosphorylation.
Calcium influx activates calcineurin, which dephosphorylates NFAT.
Dephosphorylated NFAT moves to the nucleus to promote gene transcription for T cell activation.
Immunosuppressants like cyclosporin and tacrolimus inhibit calcineurin, preventing transplant rejection.
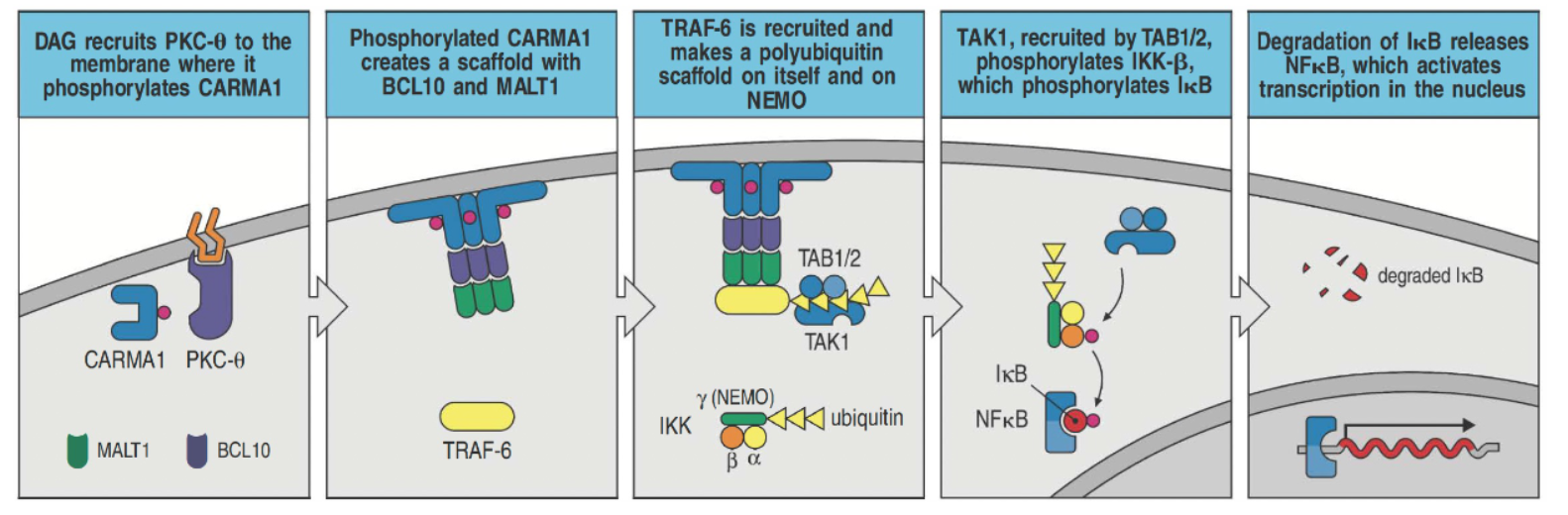
How does DAG contribute to NF-κB activation in T cells?
DAG recruits PKC-θ to the membrane, where it phosphorylates CARMA1.
Phosphorylated CARMA1 forms a complex with BCL10 and MALT1, allowing TRAF-6 binding.
TRAF-6 creates a polyubiquitin scaffold on itself and NEMO, recruiting TAK1.
TAK1, with TAB1/2, activates IKK-β, which then phosphorylates IκB.
IκB degradation releases NF-κB, allowing it to enter the nucleus and activate transcription.
PKC-θ also activates JNK and AP-1 for further transcriptional regulation.
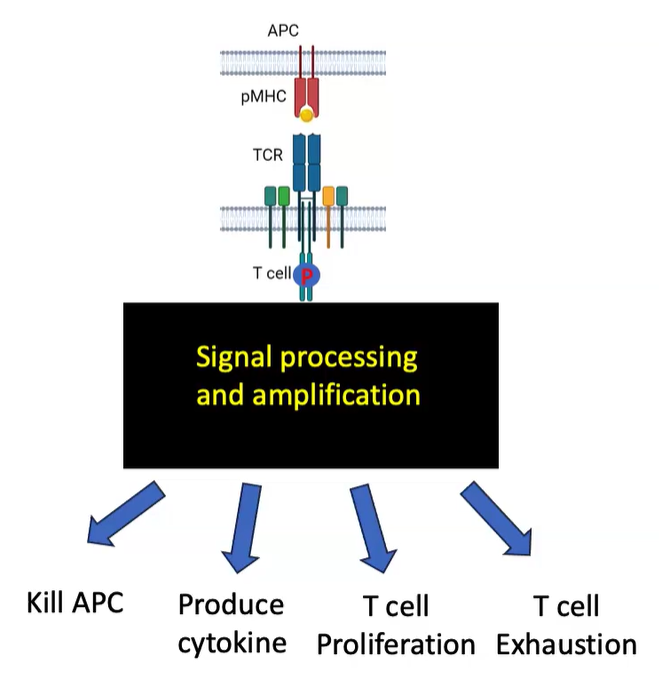
What are the possible outcomes of T cell receptor (TCR) signaling?
TCR signaling amplifies signals, enabling various T cell functions:
APC killing
Cytokine production
T cell proliferation
T cell exhaustion (under prolonged activation conditions)
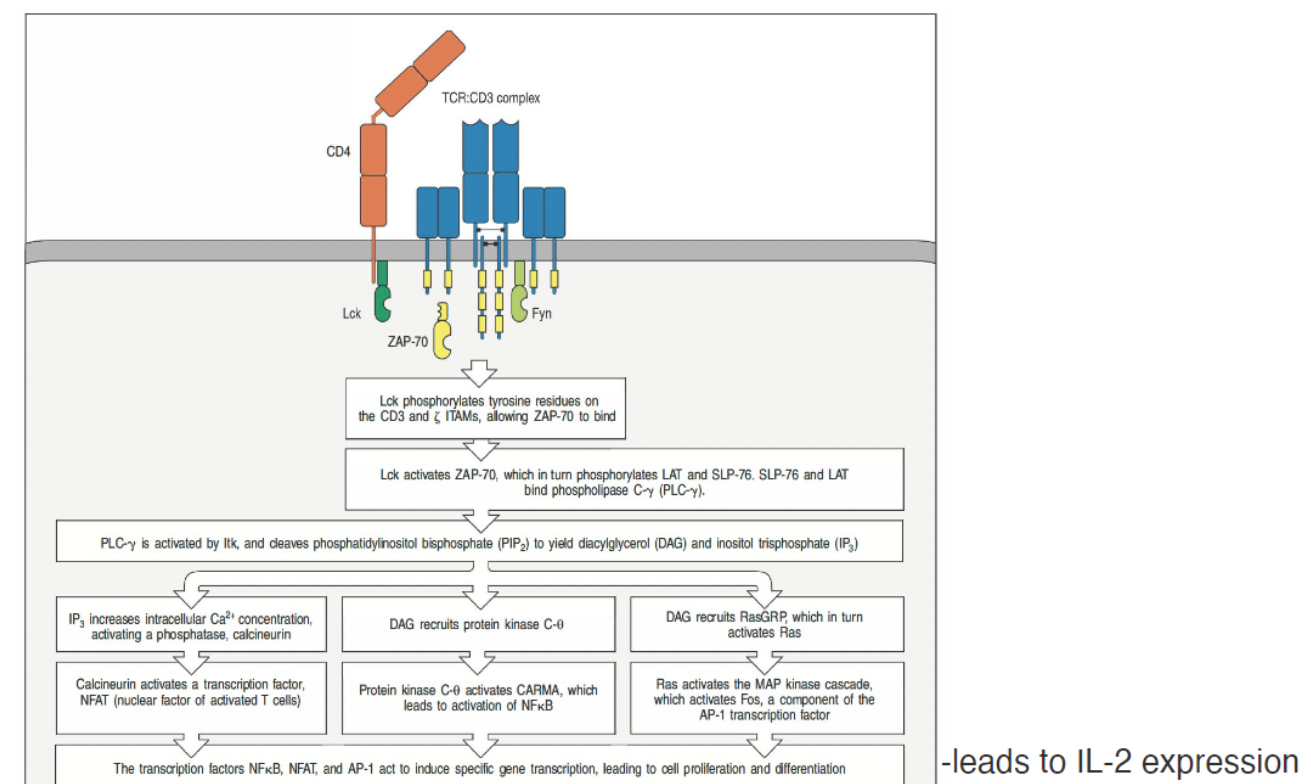
How does T cell receptor (TCR) signaling lead to T cell proliferation and differentiation?
TCR-CD4 recognition of MHC-peptide complex initiates signaling
CD4 engages Lck to phosphorylate ITAMs on TCR complex
Phosphorylated ITAMs recruit and activate ZAP-70
ZAP-70 activation leads to:
PLC-γ activation, which cleaves PIP₂ into DAG and IP₃
IP₃ releases calcium, activating:
Calmodulin and calcineurin, which dephosphorylate NFAT for nuclear entry
DAG activates PKC-θ, leading to NF-κB and AP-1 activation
Pathways together induce IL-2 expression, driving T cell proliferation and differentiation
How is the B cell receptor (BCR) activated, and what is the role of ITAMs in this process?
BCR complex is activated by antigen cross-linking.
Lacks ITAM motifs but associates with Igα and Igβ, which contain ITAMs.
Phosphorylation of ITAMs initiates downstream signaling for B cell responses.
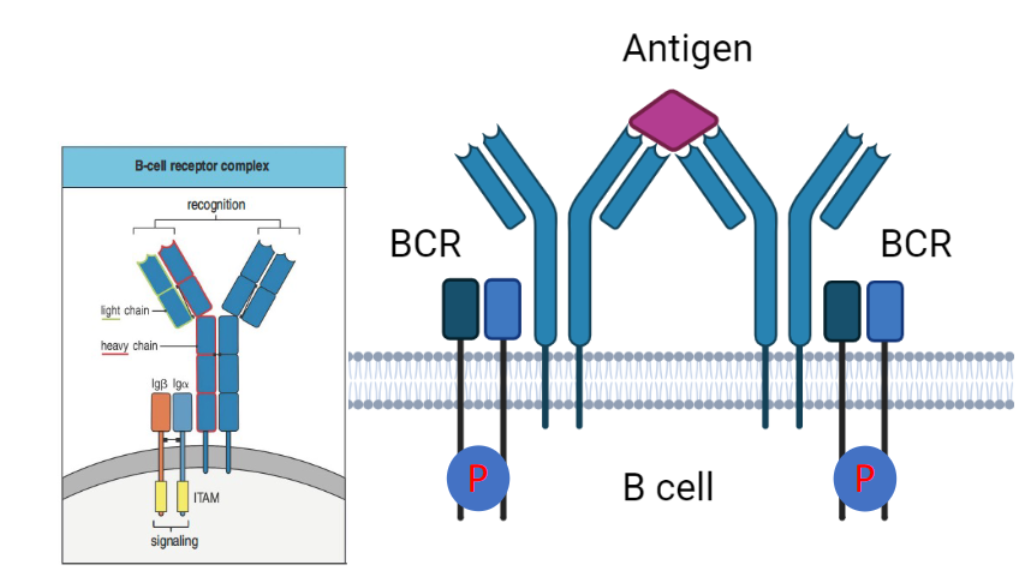
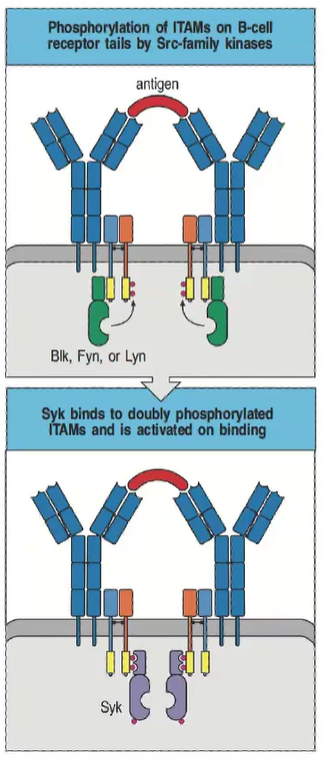
How is B cell receptor (BCR) signaling initiated, and what kinase is involved?
B cell ITAMs are phosphorylated by Src-family kinases (Blk, Fyn, Lyn).
B cells use Syk (not ZAP-70) for signaling.
Syk binds doubly phosphorylated ITAMs, activating downstream pathways.
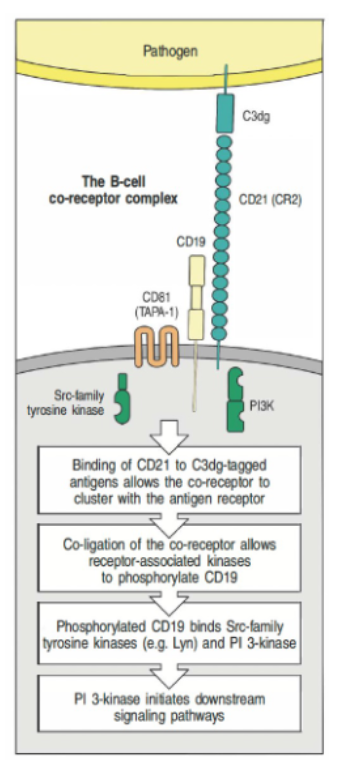
How does the B cell co-receptor complex enhance B cell receptor (BCR) signaling?
The B cell co-receptor complex (CD21, CD19, CD81) enhances BCR signaling.
CD21 binds C3dg-tagged antigens, clustering with the BCR.
Co-ligation enables Src-family kinases to phosphorylate CD19, recruiting PI3-kinase for downstream signaling.
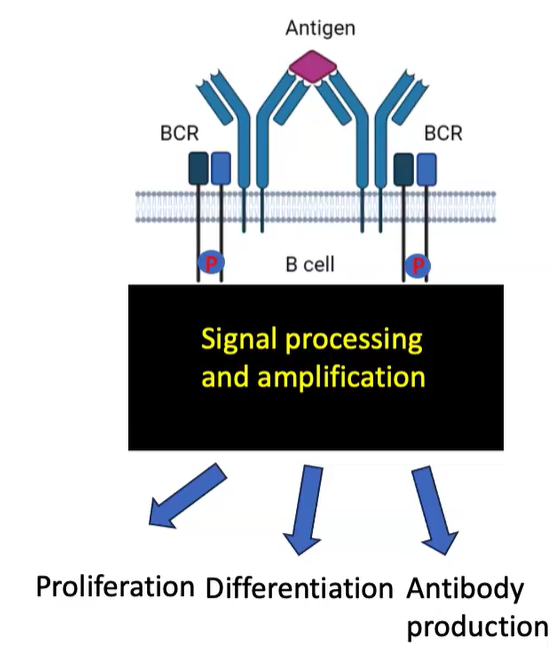
What are the outcomes of B cell receptor (BCR) activation through antigen cross-linking?
BCR activation via antigen cross-linking triggers signal processing and amplification.
Leads to B cell fate decisions: proliferation, differentiation, and antibody production.
Involves modifications like phosphorylation, lipid signaling, and ubiquitination to regulate responses.
What are the key steps involved in BCR signal integration leading to cellular outcomes in B cells?
Activation Trigger: BCR is cross-linked by an antigen.
Key Kinases: Src-family kinases (e.g., Fyn, Lyn) phosphorylate ITAM motifs on the BCR complex.
Signal Amplification: Syk kinase binds to phosphorylated ITAMs via SH2 domains, initiating further downstream signaling.
Pathway Convergence: Phospholipase C-γ cleaves PIP₂ to produce DAG and IP₃, which triggers calcium signaling.
Nuclear Activation: Transcription factors (NF-κB, NFAT, AP-1) translocate to the nucleus, resulting in B cell proliferation and differentiation.
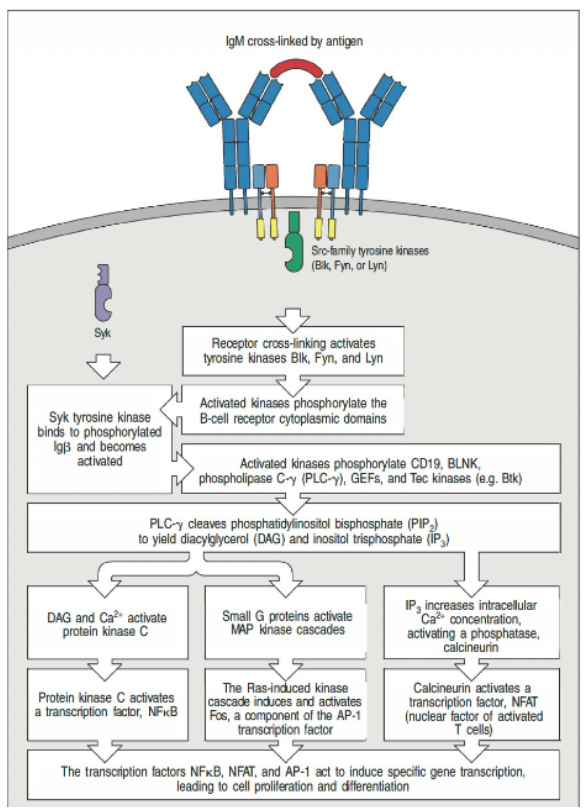
What is the role of multisubunit complexes and co-receptors in TCR and BCR activation?
Antigen receptors on lymphocytes are multiprotein complexes.
Multisubunit complexes are essential for activating TCR and BCR.
Co-receptors can either promote or inhibit TCR and BCR activation.
Protein chains in these receptors have tyrosine-containing signaling motifs called ITAMs.
Signaling is tightly regulated by ITAMs along with additional kinases and phosphatases.
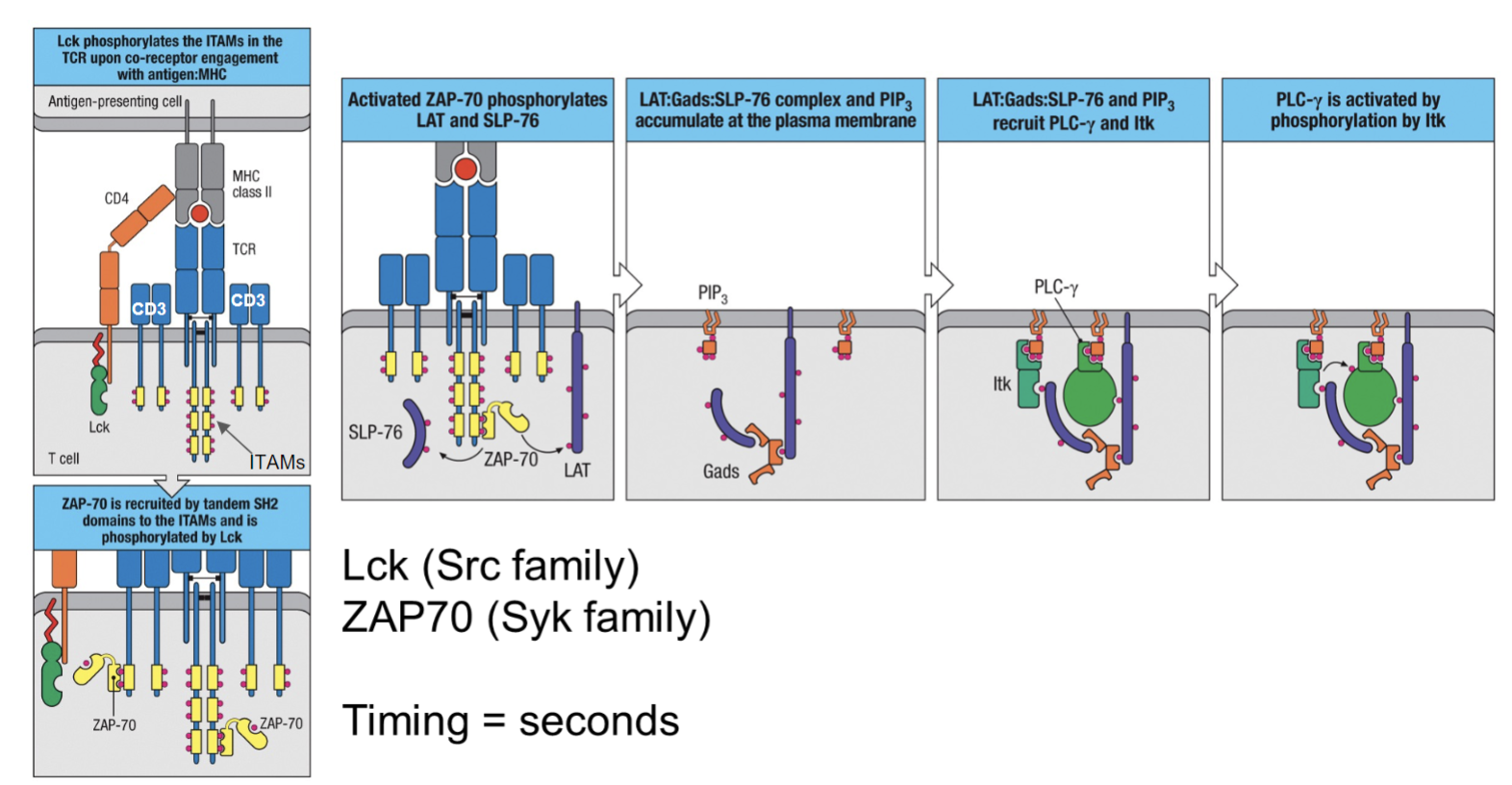
What is the sequence of events in TCR signaling involving Lck, ZAP-70, and PLC-γ?
Initial Activation: Lck (a Src family kinase) phosphorylates ITAMs in the TCR complex upon engagement with the MHC
complex.
ZAP-70 Recruitment: ZAP-70 (a Syk family kinase) is recruited to the phosphorylated ITAMs through its SH2 domains and is activated by phosphorylation from Lck.
Formation of LAT Complex: Activated ZAP-70 phosphorylates adaptor proteins LAT and SLP-76, leading to the formation of a LAT:Gads
complex.
PIP₃ Accumulation: This complex, along with PIP₃, recruits PLC-γ and Itk to the plasma membrane.
PLC-γ Activation: PLC-γ is activated through phosphorylation by Itk, initiating further downstream signaling pathways.
Timing: The process occurs within seconds.
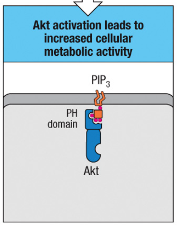
What is the role of Akt activation in T cells?
Akt activation increases cellular metabolic activity, supporting the energy demands of T cell activation and funSction.
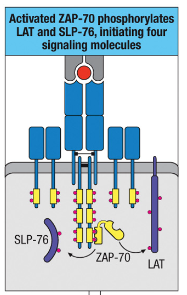
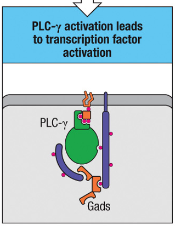
How does PLC-γ activation contribute to T cell responses?
PLC-γ activation triggers transcription factor activation, which initiates gene expression changes needed for T cell responses.
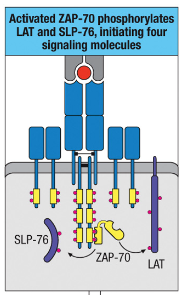
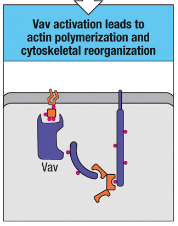
What is the function of Vav activation in T cells?
Vav activation leads to actin polymerization and cytoskeletal reorganization, allowing T cells to change shape and move, facilitating immune cell interactions.
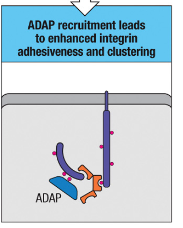
How does ADAP recruitment aid in T cell-APC interactions?
ADAP recruitment enhances integrin adhesiveness and clustering, stabilizing T cell-APC contact for effective signaling and strong cell-cell interactions.
What are the four major downstream modules following T cell activation, and what are their roles?
Akt Activation: Leads to increased cellular metabolic activity, supporting the energy demands of T cell activation and function.
PLC-γ Activation: Triggers transcription factor activation, essential for initiating gene expression changes that drive T cell responses.
Vav Activation: Leads to actin polymerization and cytoskeletal reorganization, enabling T cells to change shape and move, which is important for immune cell interactions.
ADAP Recruitment: Enhances integrin adhesiveness and clustering, strengthening cell-cell interactions and stabilizing T cell-APC contact for effective signaling.