Chem 2c Midterm 1
1/29
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
30 Terms
Coordination compounds
transition metal ions, in combination with ligands and counterions
general term for neutral compounds containing transition metals
complex ions
transition metal ions in combination with ligands
used when transition metal species have a nonzero charge
counterions
anions or cations needed to produce a compound with no net charge
coordination number
the number of nearest neighbors to the transition metal ion
typically the number of ligand surrounding the ion
ligands
lewis base (electron pair donor)
will have a lone pair of electrons ready to form a bond
has negative or neutral cha
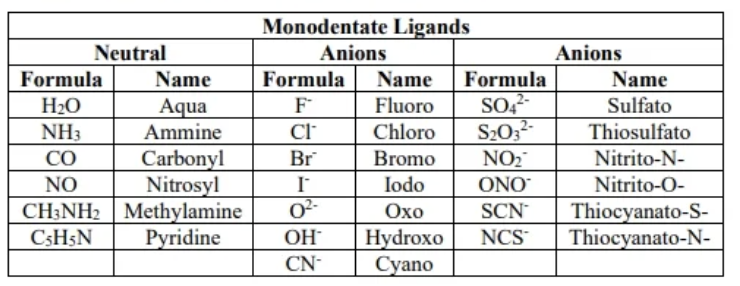
monodente
a ligand with one pair of electrons to bond
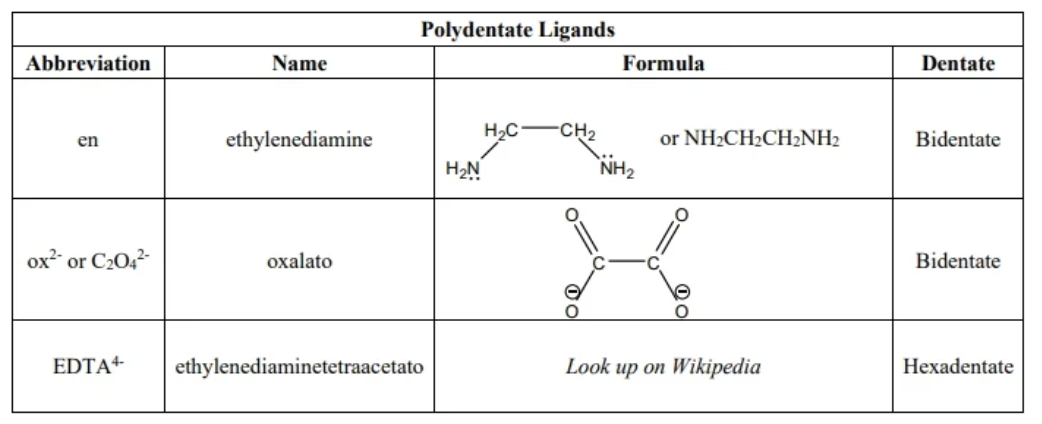
polydente
a ligand with two or more elctron pairs to bond with
monodente ligands (examples)
polydente ligands (examples)
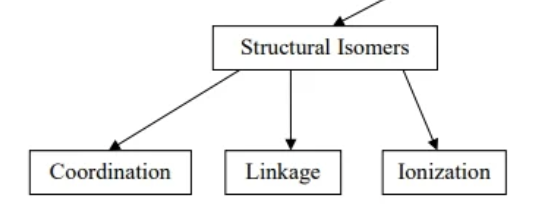
ionization isomers
a structural isomer where a ligand and counter ion switch
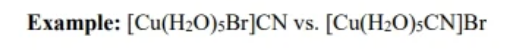
coordination isomers
structural isomer where the transition metals of a bi-metallic species switch ligands
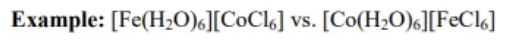
linkage isomers
structural isomers where a multi-atom ligand connects to the transition metal through different atoms
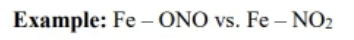
geometric isomers
a stereoisomer where the arrangement of ligands are either neighboring (cis or fac) or across (trans or mer) from each other
structural isomers
compounds that can have different connectivity
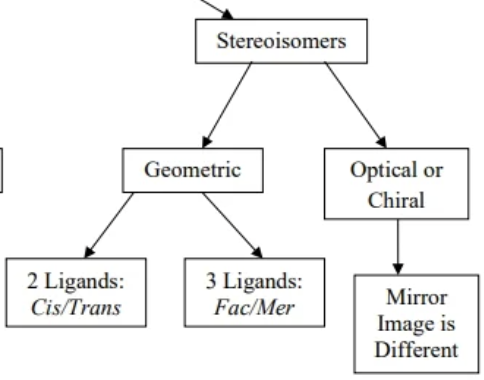
stereoisomers
compounds that can have different arrangement of atoms in space
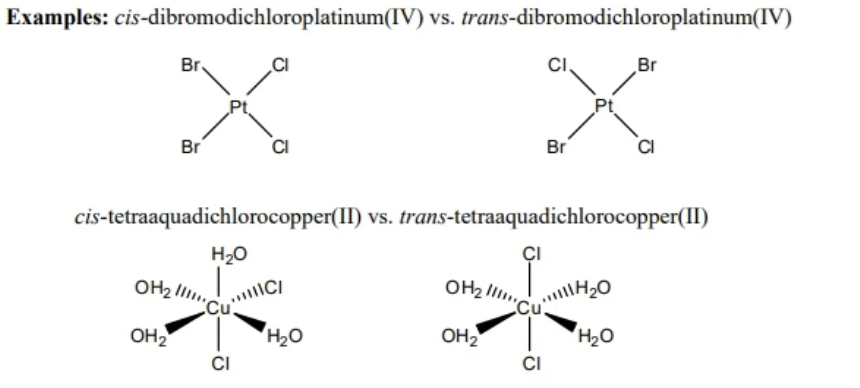
cis/trans isomerism
occurs only in square planar and octahedral complexes
2 identical ligands are either neighboring (cis) or opposite (trans)
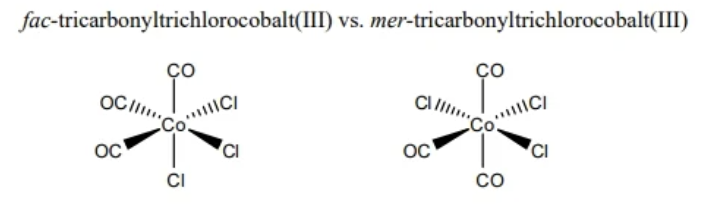
fac/mer isomerism
occurs only in octahedral complexes
3 identical ligands are either neighboring (fac) or across (mer)
optical isomer
subcategory of a stereoisomer where the arrangement of atoms results in a non superimposable mirror image
also called chiral or optically active
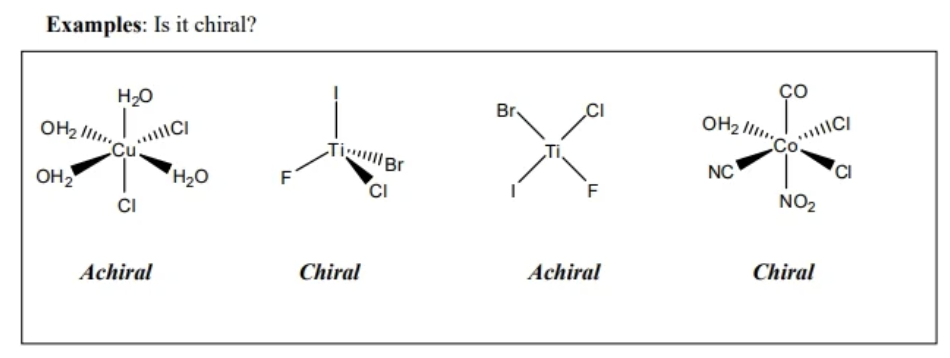
enantiomer
the set of mirror image optical isomers
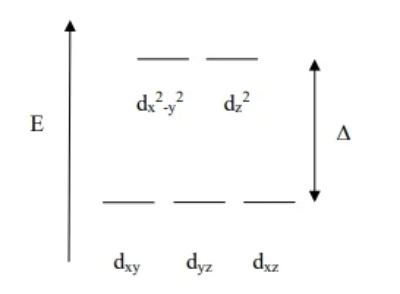
Octahedral Crystal field theory diagram
Opposite of tetrahedral
Tetrahedral Crystal field theory diagram
Opposite of octahedral diagram
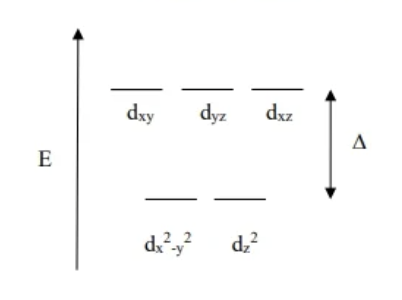
Square planar Crystal field theory diagram
Never fill top level unless all 10 e- are present
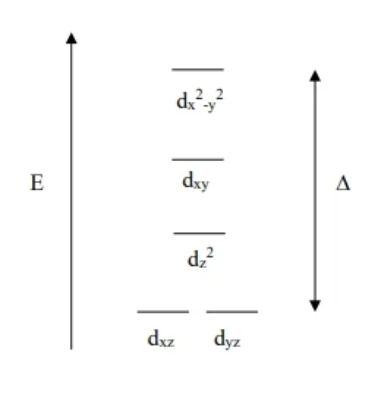
Linear Crystal field theory diagram
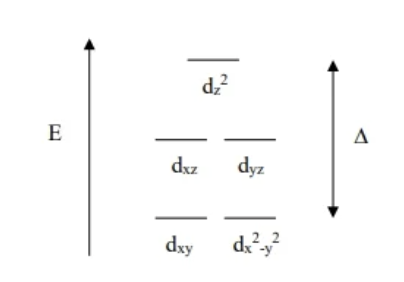
Crystal Field theory electron filling
Strong field:
low-spin
orbitals far apart (large Δ)
bottom level fills first before moving to the next level
Weak field:
high spin
orbitals close together (small Δ)
all orbitals fill singly first
How to determine Δ
