Chapter 2 - Atoms, Isotopes, Ions, & Molecules
1/16
Earn XP
Description and Tags
Biol 111
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
17 Terms
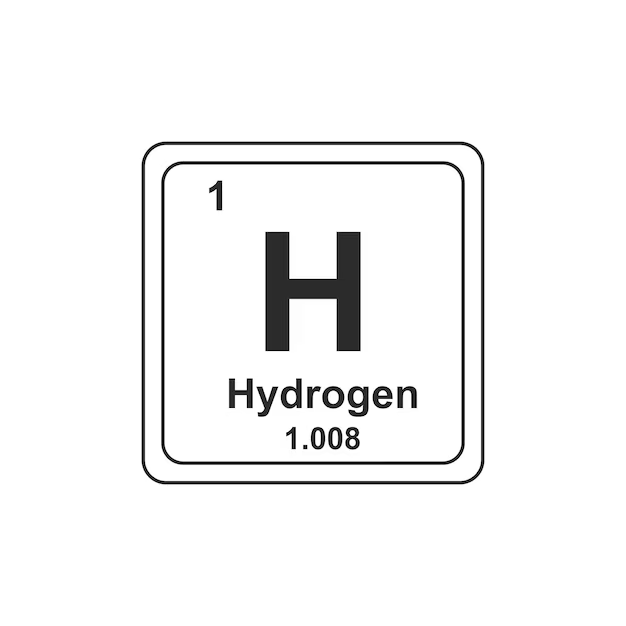
() are substances that cannot be broken down to other substances by chemical reactions; consists of a certain kind of () that is different from those of other elements; designated by a 1 or 2 letter ().
elements, atom, symbol,
The smallest unit of matter that still retains the properties of an element is the ()
atom
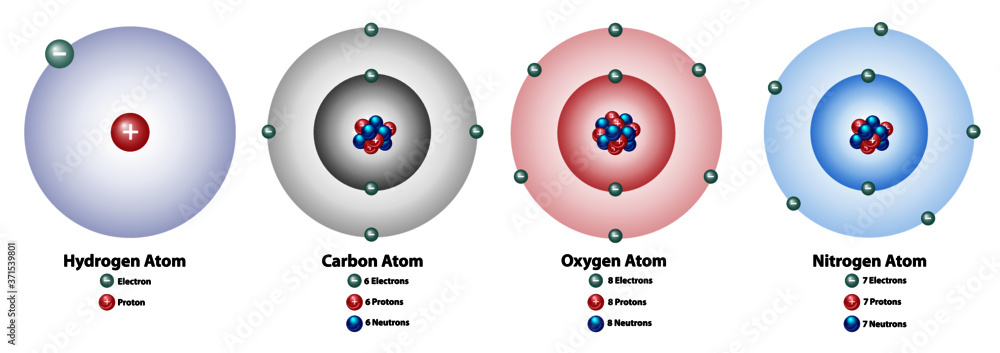
Oxygen, carbon, hydrogen, and nitrogen are all () to life; they make up to 96% of () matter
essential, living
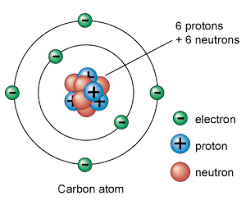
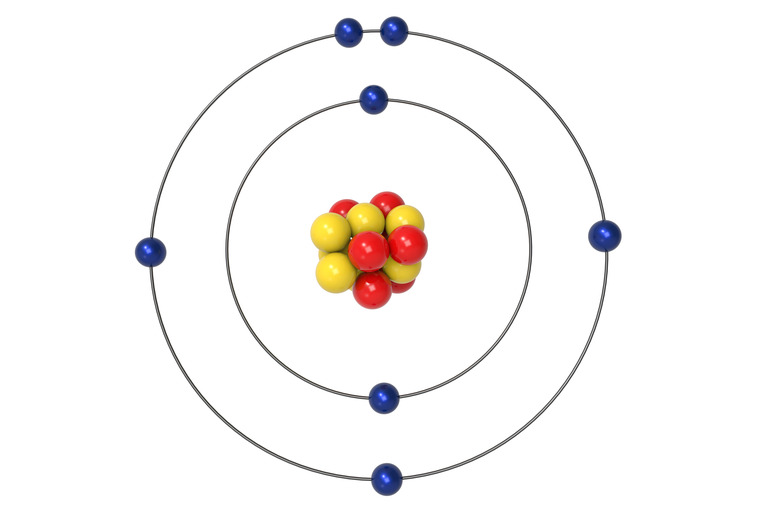
The nucleus of an atom contains () and (), while the cloud contains (); they have a positive, neutral, and negative charge respectively.
protons, neutrons, electrons,
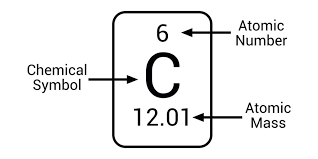
The atomic number is equal to the number of (), it defines each element; the atomic mass is equal to the sum of proton and () mass; each proton and neutron have close to 1 Dalton/amu in mass; electrons have a mass of 1/2000 Dalton which is ().
protons, neutrons, negligible
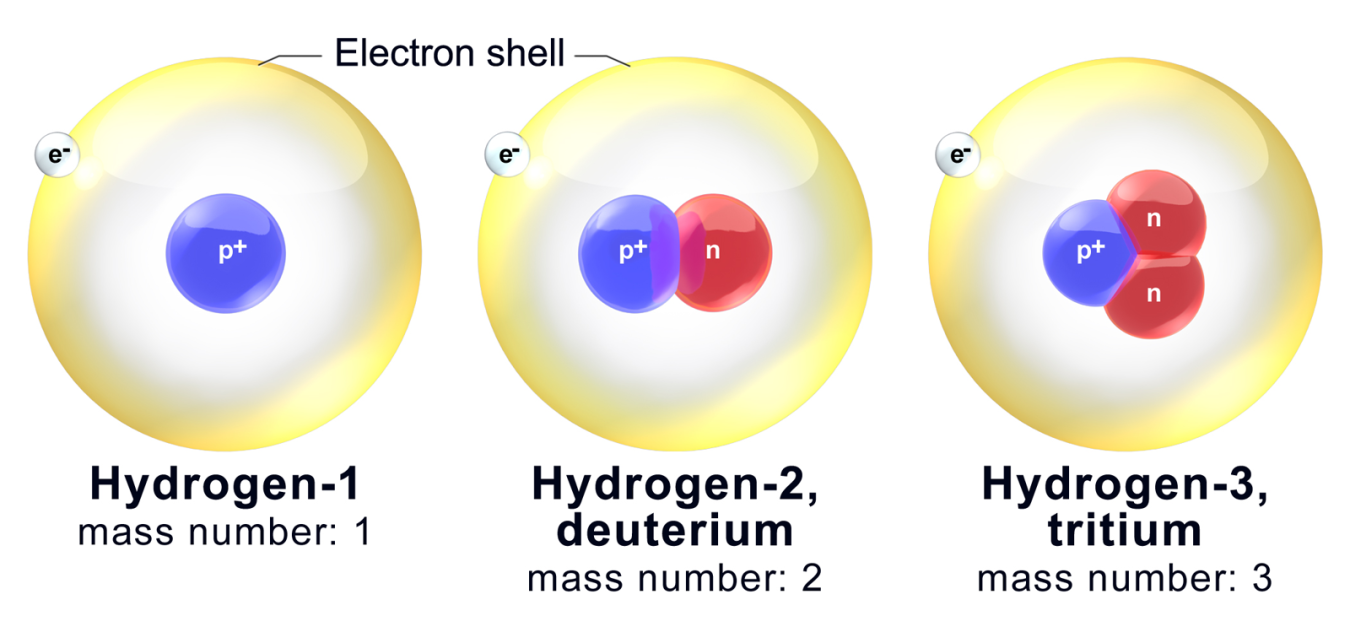
An () is an element that differs in the number of neutrons; radioactive isotopes () neutrons, protons, and electrons.
isotope
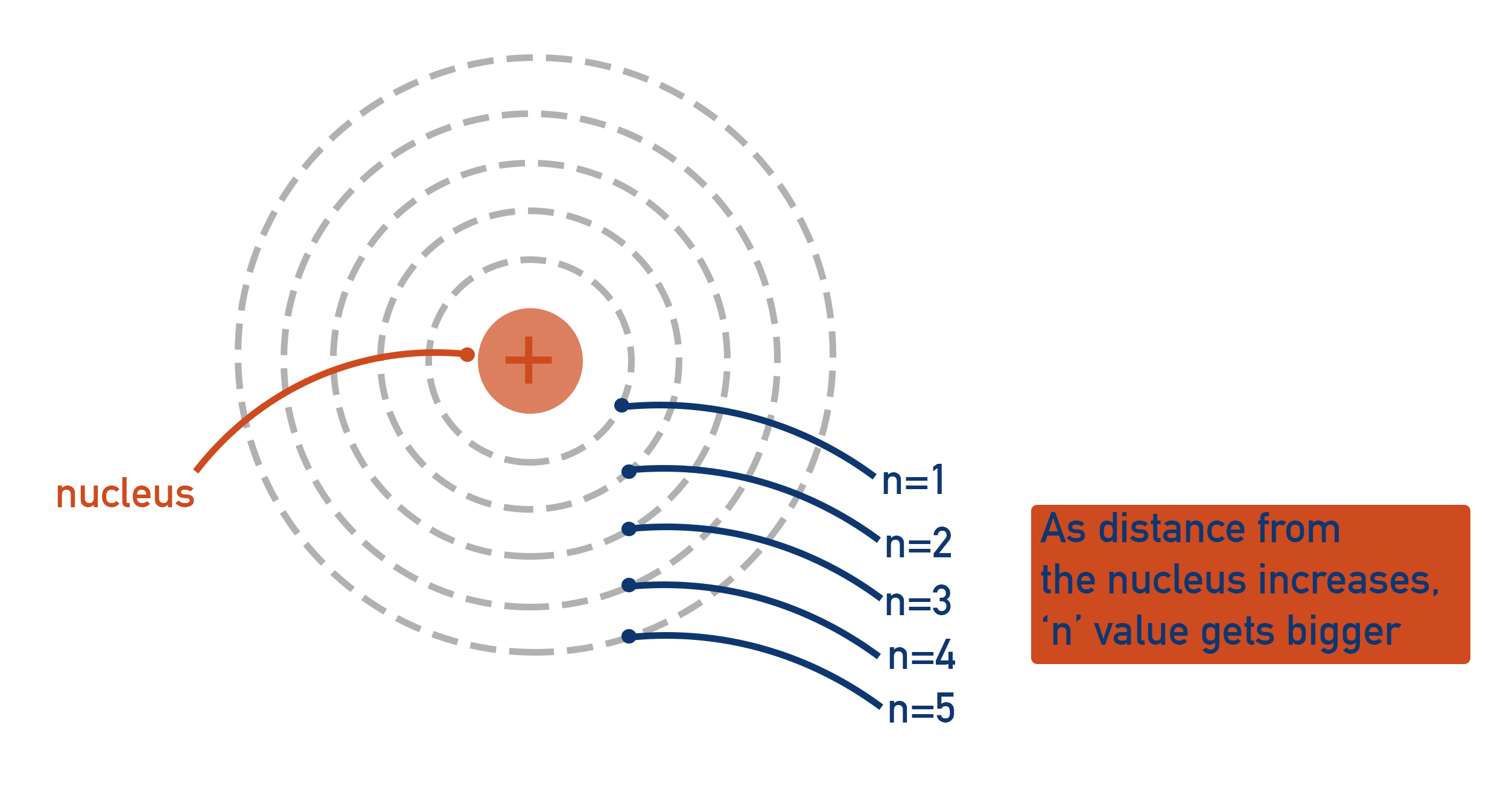
The chemical behavior of an atom is defined by its () configuration and distribution; they vary in the amount of () they possess; usually found at their () possible energy state; electrons fill () closest to nucleus first, then those further away in order; typically equal to the number of () in an atom.
electron, energy, lowest, orbitals, protons
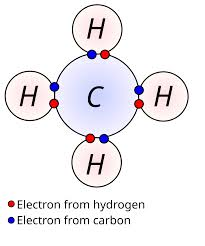
() electrons are the electrons in the outermost shell of an atom; atoms interact with other atoms to () their valence shell; an atom with a full valence shell is (); valency is the number of () electrons; a
valence, complete, unreactive, unpaired
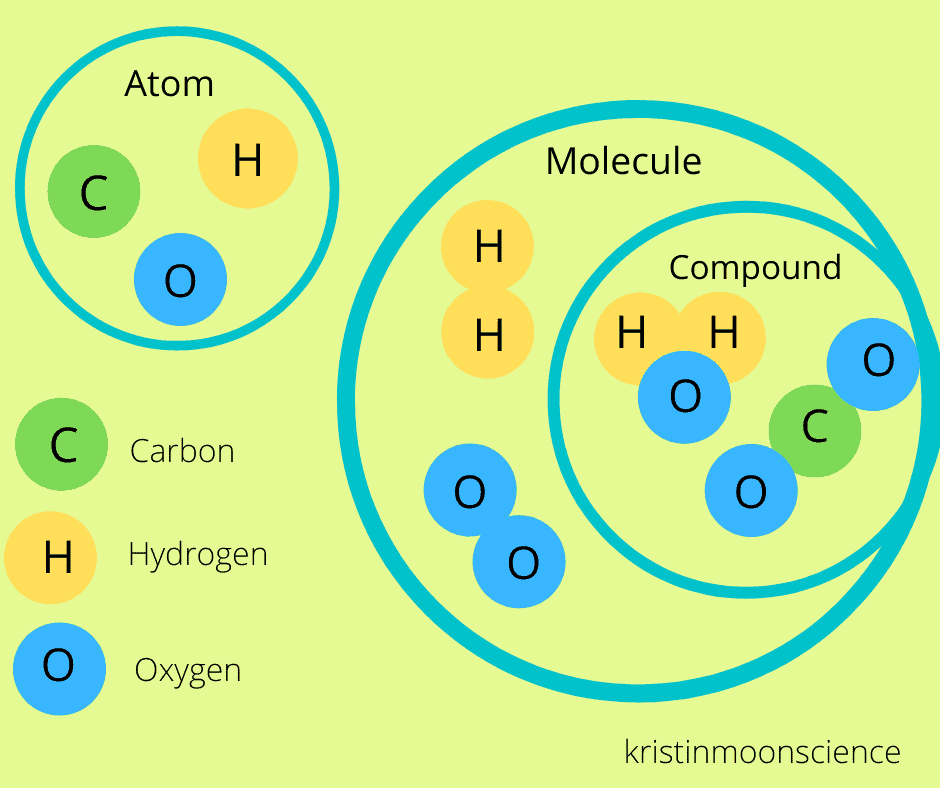
A () is 2 or more atoms covalently bonded together; a () is 2 or more different elements bonded in a fixed ratio; () reactions are changes in distribution of electrons between atoms; form and function of molecules depend on the chemical () formed.
molecule, compound, chemical, bonds,
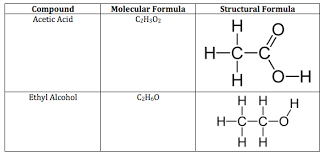
A () formula indicates the number and types of atoms in a molecule; a () formula has a line to represent each pair of shared electrons.
molecular, structural
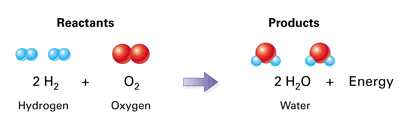
Bonds of () must be broken, then the new bonds of the () can form; () chemical reactions happen when the reactants are converted to products but some products can be converted back; () reactions proceed in one direction until all reactants are used up.
reactants, product, reversible, irreversible
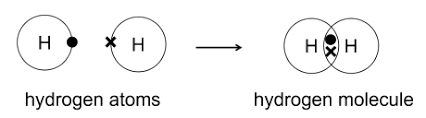
The sharing of a pair of valence electrons that results in a biological strong bond is a () bond; this produces stable, relatively unreactive ().
covalent, molecules
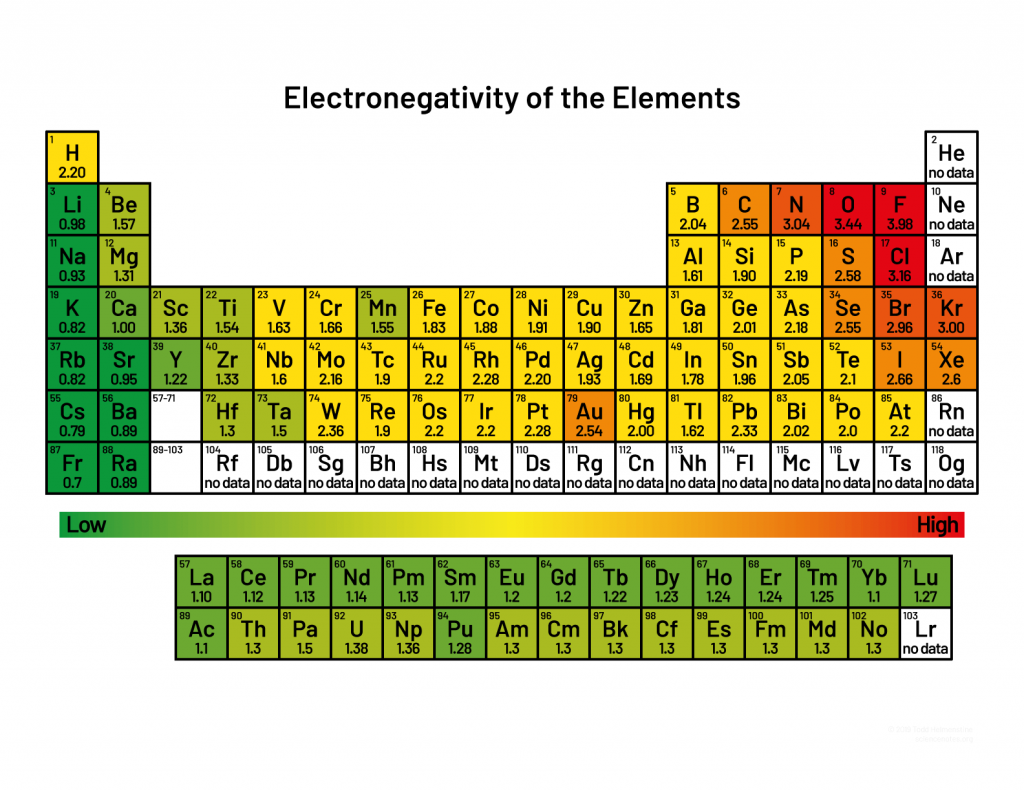
() is the attraction of a particular kind of atom to the electrons in a covalent bond; more () in the nucleus means more electronegative, more electrons in the cloud means () electronegative, the greater the distance of the outer electrons from the distance the () electrongative.
electronegativity, protons, more, less
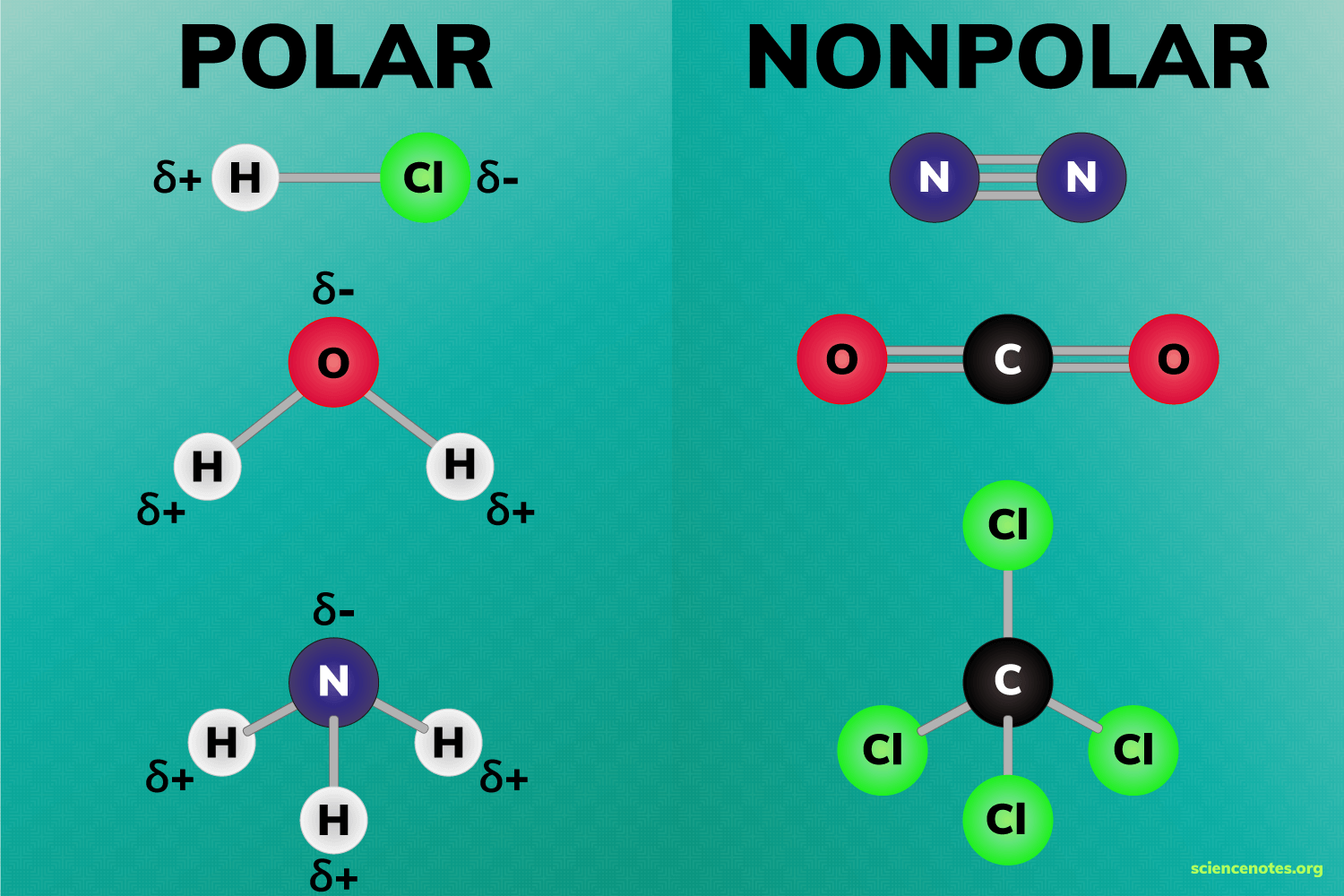
() covalent bonds happen when the atoms have similar electronegativities and there is an equal sharing of electrons; () covalent bonds happen when the atoms have different electronegativities and there is an unequal sharing of electrons.
nonpolar, polar
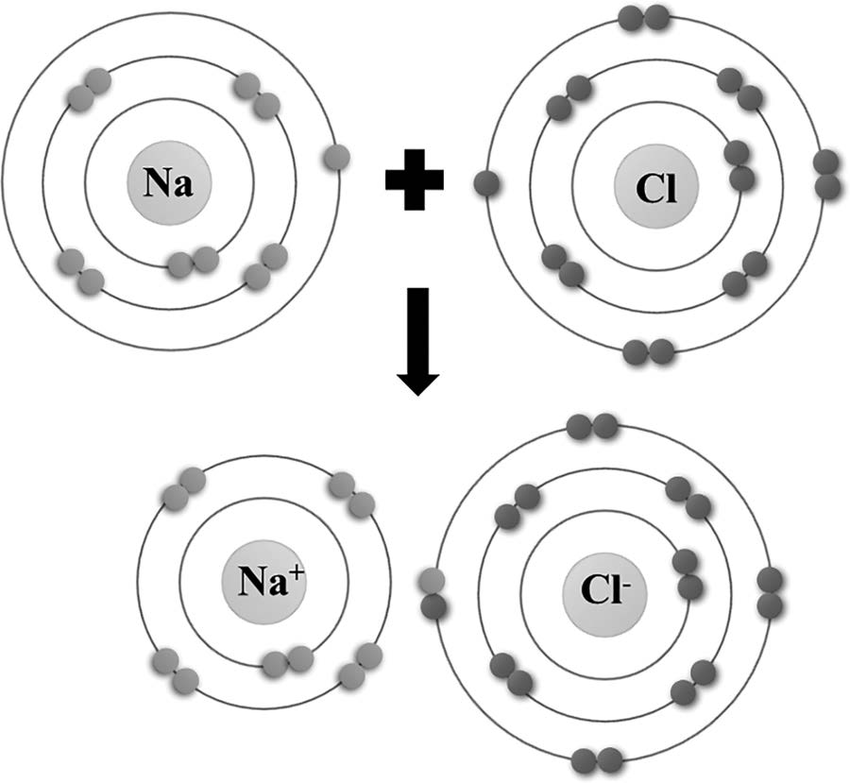
An () bond is the electrical attraction between the charges of anions and cations; atoms are so electronegative that they () electrons from their bonding partners; electron transfer to complete the valence shells causes the creation of (); anions are () charged while () are positively charged.
ionic, transfer, ions, negatively, cations
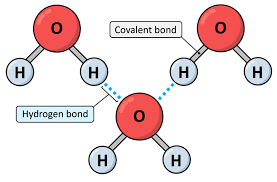
() bonds happen when a hydrogen atom covalently bonded to a more () atom in a molecule is also attracted to another electronegative atom/atom in another molecule; these bonds are () and are common between water and DNA.
hydrogen, electronegative, weak
The () of a molecule is determined by the positions of its atoms’ valence orbitals; determines how biological molecules recognize and () to one another with specificity; allow structures to self ().
shape, responds, assemble