Analytical Chemistry Test 2
1/148
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
149 Terms
Explain the process of molecular absorption in terms of “states.”
Paired electrons reside at the ground state (S0). When light is absorbed, an electron will jump to a higher electron state (S1, S2, etc.). Within the excited states is various vibrational states to which the electron can move.
What is the effect of intermolecular forces on molecular absorbance spectra?
Intermolecular forces broaden peaks. Therefore, environment effects the peaks. A system in gas phase will produce strong peaks. A system in water will produce broad peaks. A system in cyclohexane will produce peaks in the middle.
What does each variable mean: A=-log(P0/P)?
A= absorption
P0=incident light
P=transmitted light
Why is it important to measure P0 as a blank?
to correct for scattering and reflection losses
Name two light sources for UV-Vis absorption and what spectral range it covers.
1) deuterium arc lamp - UV
2) tungsten lamp - vis/NIR
Name the different types of cuvettes, the range of spectrum they cover, and their cost. What other factors should be considered when picking a cuvette?
1) Quartz - UV, vis, NIR - $90
2) Optical glass - partial UV, vis, NIR - $40
3) Polystyrene - partial UV, Vis - $0.2
4) UV acrylic - UV, vis - $0.8
Other factors: chemical resistance and physical durability
One way to increase the absorbance of your sample.
Increase the path length
Name the four causes of deviations from Beer-Lambert’s law and how to limit each of them.
1) concentration - stay below 0.01 M
2) acids/bases - control pH using excess H+ or OH-
3) wavelength - measure at peak and use *picture
4) “stray light” at wrong wavelength causes error at high absorbance - note absorbance limit
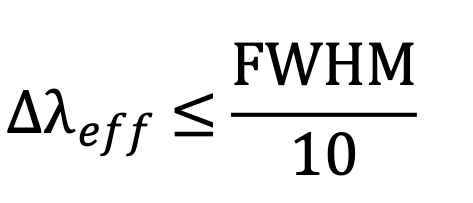
Explain the effect of stray light at high absorbances.
When a sample absorbs a lot of light, less light is transmitted to the detector. Added light reaching the detector (stray light) can result in a lower absorbance value than the real absorbance.
Draw a single beam instrument and a double beam instrument.

Why is a chopper important in a double beam instrument?
Reduces uncertainty in P0
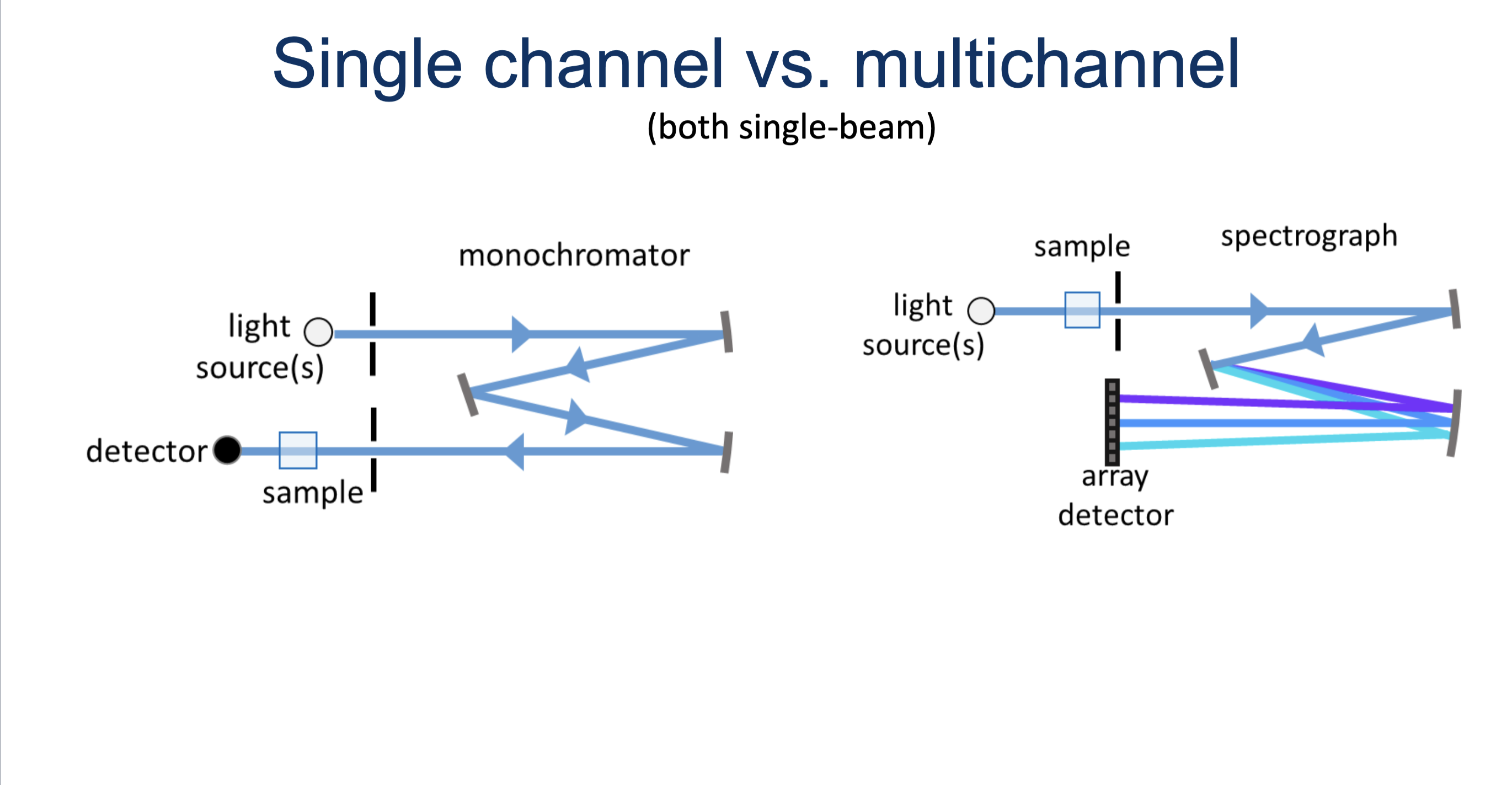
Draw a single-channel and multi-channel instrument. Note key differences.
Single-channel : sample refracts then enters sample and goes straight to detector, single beams
Multi-channel : light hits sample first them refracts and enters detector, detects and array of light rather than single beam
What are the strengths and weaknesses of molecular absorbance techniques?
Strengths
wide applicability (can absorb many chemicals)
fast
inexpensive
Weaknesses
limited selectivity (overlap of broad absorbance)
higher LOD than luminescence
Define luminescence.
Emission of light after nonthermal excitation.
Define photoluminescence and give examples.
emission of of light after excitation by light
examples: fluorescence, phosphorescence
Define chemiluminescence
emission of light after excitation by chemical reaction
Describe the different steps of fluorescence.
1) absorption = molecule absorbs light and is excited from S0 to S1/2
2) vibrational relaxation = molecule relaxes to lower vibrational states by releasing nonradioactive energy
3) internal conversion = molecules transfer energy within itself and virbrationaly relaxes to the S1 state
4) fluorescence = molecule returns to lowest energy state, S0, by emitting a photon
Describe the different steps of phosphorescence.
1) absorption = molecule absorbs light and is excited from S0 to S1/2
2) vibrational relaxation = molecule relaxes to lower vibrational states by releasing nonradioactive energy
3) internal conversion = molecules transfer energy within itself and virbrationaly relaxes to the S1 state
4) intersystem crossing = transition from S1 to T1 (triplet state), facilitated by spin-orbit coupling
5) phosphorescence = molecule returns to lowest energy state, S0, by emitting a photon, slower than fluorescence
what is internal and external conversion?
Internal conversion = molecule transforms energy to vibrational energy within itself and moves to lower electronic state
external conversion = molecule transfers energy to another molecule and moves to a lower electronic state
Draw a fluorometer and describe the selectivity of it.
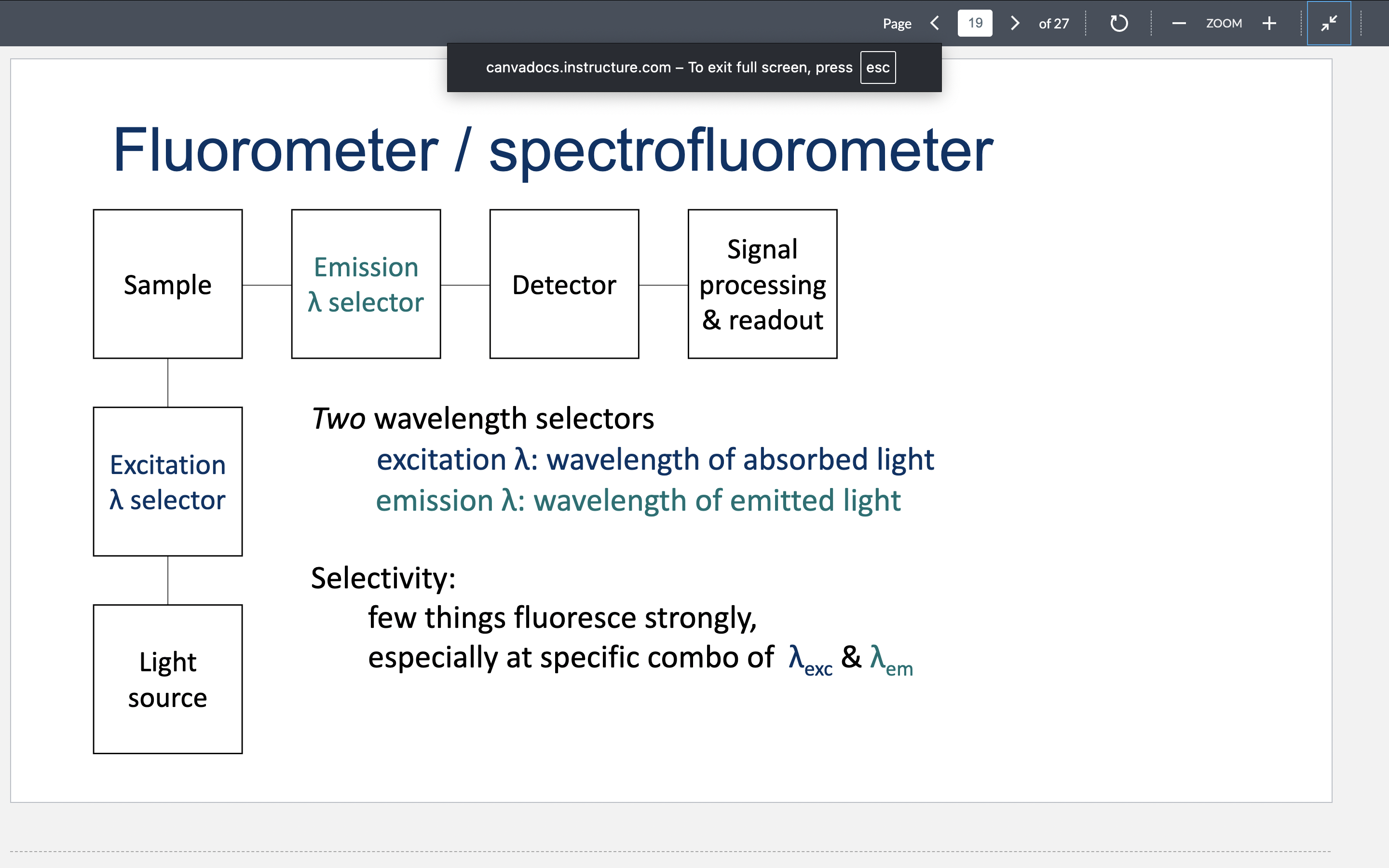
What is the relationship between concentration, absorbance, and fluorescence in a fluorometer?
At high concentrations, there is nonlinear fluorescence and good S/N ratio for absorbance. At low concentrations, there is linear fluorescence, poor S/N ratio for absorbance (absorbance is small), and high S/N ratio for fluorescence (no background interference).
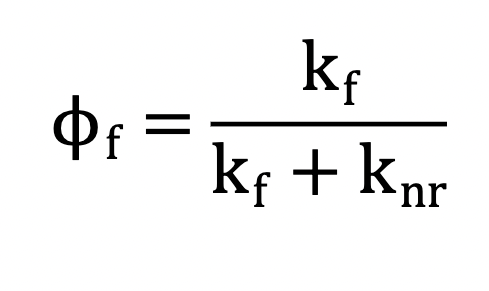
What does each variable mean? What exactly is it measuring?
Measures the efficiency of fluorescence.
O = fluorescence quantum yield
Kf = fluorescence rate constant
Knr = sum on nonradiative rate constants
Why do most molecules have weak fluorescence and what can be done to make it more efficient?
Because the non-radiative rate constants are much higher than the fluorescence rate constants. You can increase the fluorescence rate constant and decrease the non-radiative rate constants.
what is fluorescence lifetime and how does time effect fluorescence intensity? What is needed to measure it?
The average time a molecule stays in the excited singlet state before emitting a photon. The intensity of fluorescence decreases over-time. A short light pulse and a fast detector is needed to measure fluorescence lifetime.
What is dynamic (collisional) quenching and what occurs as a result of it?
When a fluorophore transfers energy to another molecule (quencher) after excitation. It decreases the fluorescence (as a result of increasing the Knf) and decreases the fluorescence lifetime.
What is static quenching and what effect does it have?
A fluorophore complexes with a quencher before excitation. Fluorescence decreases (as a result of a decrease in F). Fluorescence lifetime is not effected.
What is the relationship between the concentration of a quencher and fluorescence?
Increase in concentration decreases intensity
what is an efficient fluorescence method for target DNA and how does it work?
Using molecular beacons and quenching. The molecular beacon binds to to the complementary DNA sequence, separating the fluorophore from the quencher, therefore causing fluorescence.
How can quenching be used to study biomolecule folding, DNA binding, and membrane penetration?
By measuring the distance between the fluorophore and the quencher
Name types of vibrational spectroscopy.
Absorption and scattering
What are the different types of scattering and how are they different?
1) rayleigh : photon excites to virtual state and returns to same vibrational level is started at (no energy difference)
2) stokes shifted raman : photon excites to virtual state and returns to a different vibrational level (energy lost)
3) anti-stokes shifted raman : molecule starts in excited vibrational state and returns to ground vibrational state (energy is gained)
Relationship between wavelength of light source and scattered wavelength and raman shift.
The light scattered by the sample depends on the wavelength of the light source. However, Raman shift is independent of the wavelength of the light source.
How can raman and IR spectra look different?
For symmetrical molecules, raman active vibrations will be IR inactive and vice versa. This is due to different selection rules based on quantum mechanics.
Difference between IR and raman?
IR = absorption, raman = scattering
What are some challenges with IR instrumentation and how can the Michelson interferometer help?
Challenges
noisy detectors
narrow absorbance bands (deviations from Beer’s Law)
Michelson interferometer
multiplex
resolution (mirror movement)
What type of wavelength separators are used in raman instrumentation and what are characteristics of each?
Interferometers = longer wavelength laser, multiplex advantage for noisy IR detectors
Spectrograph w/ array detector = short wavelength laser, less noisy UV/vis/NIR detectors
Is IR or Raman more useful for aqueous solutions and why?
Raman because water absorbs strongly in the IR region.
Advantages and disadvantages of short wavelength lasers.
Advantages
Raman shift is independent of source wavelength
scattering intensity is low
allows larger range of raman shift
Disadvantages
fluorescence background
photodecomposition
Advantages and disadvantages of long wavelength lasers.
Advantages
raman shift is independent of source wavelength
no fluorescence background
no photodecomposition
Disadvantages
scattering intensity it high
smaller range of raman shift
What is linearly polarized light?
Equal mix of clockwise and counterclockwise circularly polarized light (equal amplitute, out of phase)
How is linearly polarized light effected by enantiomers?
Enantiomers have different refractive indices for clockwise and counterclockwise light. Due to this, one is moving faster than the other causing a phase shift, rotating the orientation of the electric field.
What is a polarimeter and how does it work. Draw a diagram of one.
A polarimeter is used to measure the angle of rotation when linearly polarized light passes through an optically active substance. First, a polarizer converts light into polarized light. Then, the polarized light hits the sample. This light then travels to the analyzer (rotating polarizer) where the amount of light that has rotated is measured. The light then reaches the detector.

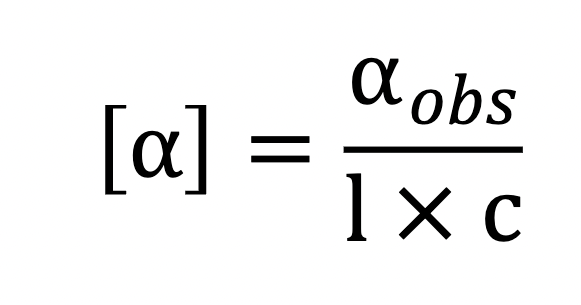
What does each variable mean?
alpha = specific rotation
alpha obs = measured optical rotation
pathlength (dm)
concentration (g/mL)
What two things can polarimetry measure?
enantiometric excess and optical purity
What is circular dichroism? What is its effect and what problems result from it?
Chiral molecules absorb left- and right- circularly polarized light. One is absorbed more preferentially than the other, resulting in an ellipse. This makes these samples hard to measure directly. CD increases with path length and concentration.
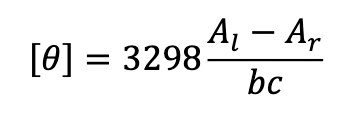
What does each variable mean?
theta = ellipticity
Al = absorbance of counterclockwise (left) light
Ar - absorbance of clockwise (right) light
b = pathlength (cm)
c = concentration (mol/L)
Draw a circular dichroism instrument. Explain how it works.
The monochromater selects a specific wavelength for the light to absorb. The polarizer polarizes the light along a fixed direction (45°), preparing it for modulation. The photoelastic modulator rapidly alternates the polarization of light between left- and right-circular (or elliptical) polarization, essential for sensitive CD measurements. Finally, the light passes through the sample and its transmitted to the detector.
![<p class="my-2 [&+p]:mt-4 [&_strong:has(+br)]:inline-block [&_strong:has(+br)]:pb-2">The monochromater selects a specific wavelength for the light to absorb. The polarizer polarizes the light along a fixed direction (45°), preparing it for modulation. The photoelastic modulator rapidly alternates the polarization of light between left- and right-circular (or elliptical) polarization, essential for sensitive CD measurements. Finally, the light passes through the sample and its transmitted to the detector. </p><p></p>](https://knowt-user-attachments.s3.amazonaws.com/48632e64-4d40-495c-8b00-1218d0754546.png)
What is befringement?
Different refractive indices for vertical and horizonal polarization.
How does a photoelastic modulator work?
45 degree polarized light (in phase) contains vertical and horizontal polarization. One of these is delayed by ¼ wavelgnth resulting in circularly polarized light (out of phase). The PEM rapidly shifts birefringence (alternates the polarization of left- and right-circular polarization).
Advantages and Disadvantages of UV-CD
Advantages
fast
inexpensive
fairly sensitive
Disadvantages
limited structural information
Draw the general layout of a mass spectrometer.
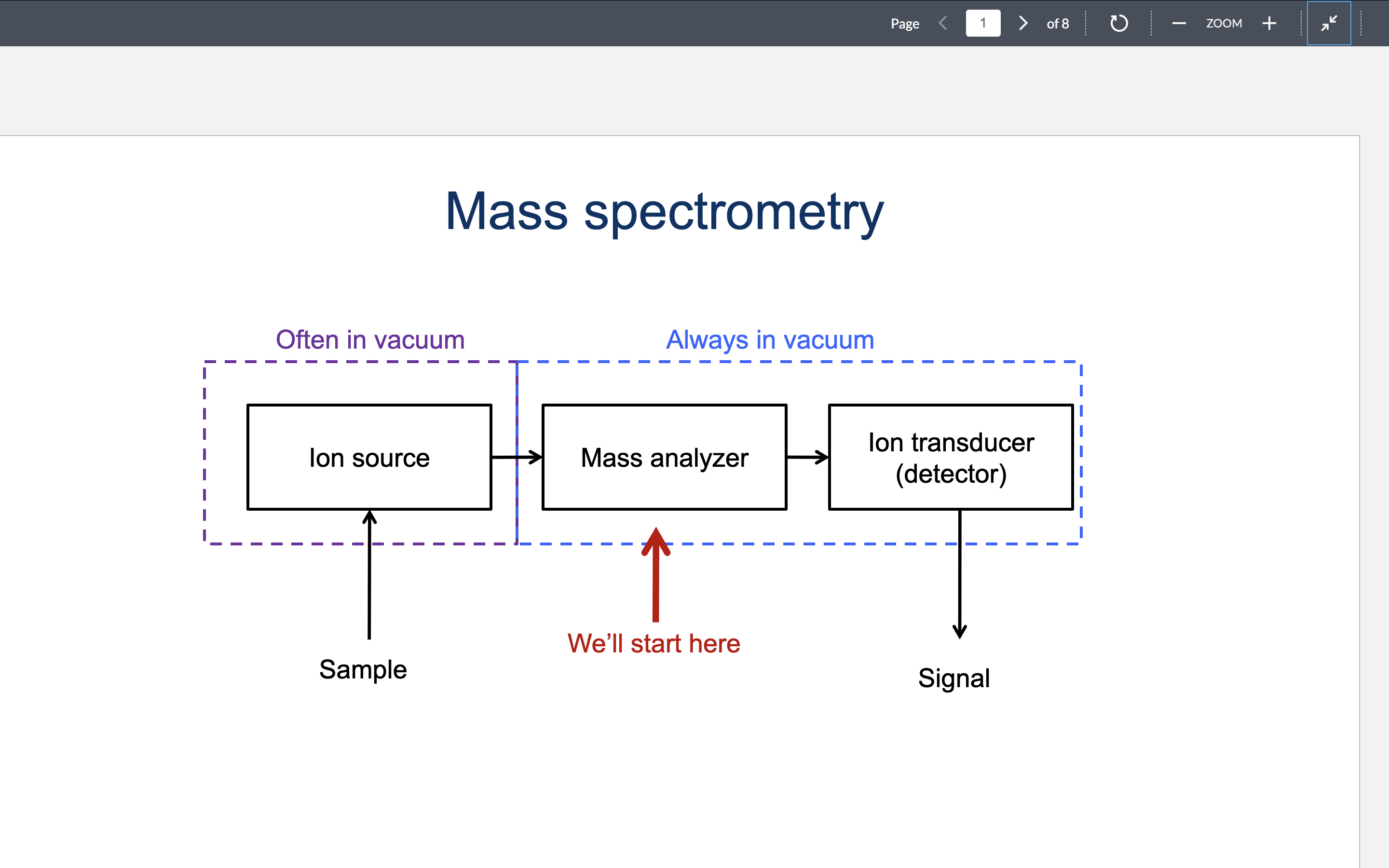
Steps of mass spec.
1) ionize molecule (typically -1/+1)
2) accelerate ion through electrical field
3) measure m/z
M/Z units
M = mass (daltons)
Z = charge (multiples of proton charge)
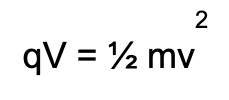
What does each variable mean? What are both sides also equal to?
q = charge
V = voltage
m = mass
v = velocity
Both sides are equal to energy (E).
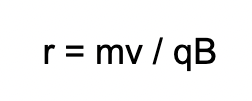
What does each variable mean?
r = radius (of circular path)
m = mass
v = velocity
q = charge
B = magnetic field strength
What is the relationship between M/Z and electric/magnetic field?
When an ion enters an electric field, it is accelerated. When it enters a magnetic field, its motion is bent into a circle. Heavier ions bend less and therefore, have a larger radius and higher M/Z ratio. Smaller ions have small radii and lower M/Z.
What is the mean free path, what does it effect, and how does it change with pressure?
Mean free path is the average distance an ion travels without collisions. It changes the velocity of the ions. An increase in pressure results in more things to collide with, decreasing the mean free path.
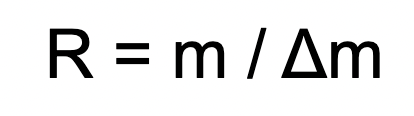
What does each variable mean?
R = resolving power
m = m/z of an ion
change in m = the smallest difference in m/z the instrument can distinguish between two peaks
What is the significance of high resolution (resolving power) in mass spec?
The “exact mass” (molecular formula) can be determined and measurements can be made in the presence of nearby interferents.
What is a magnetic sector and how does it work? Draw one.
A magnetic sector is a type of mass analyzer. It uses a magnetic field to separate ions based on M/Z. The magnetic field bends the ion’s path creating a curvature. If the radius of curvature of the ion equals the radius of curvature of the instrument, the ion is detected.
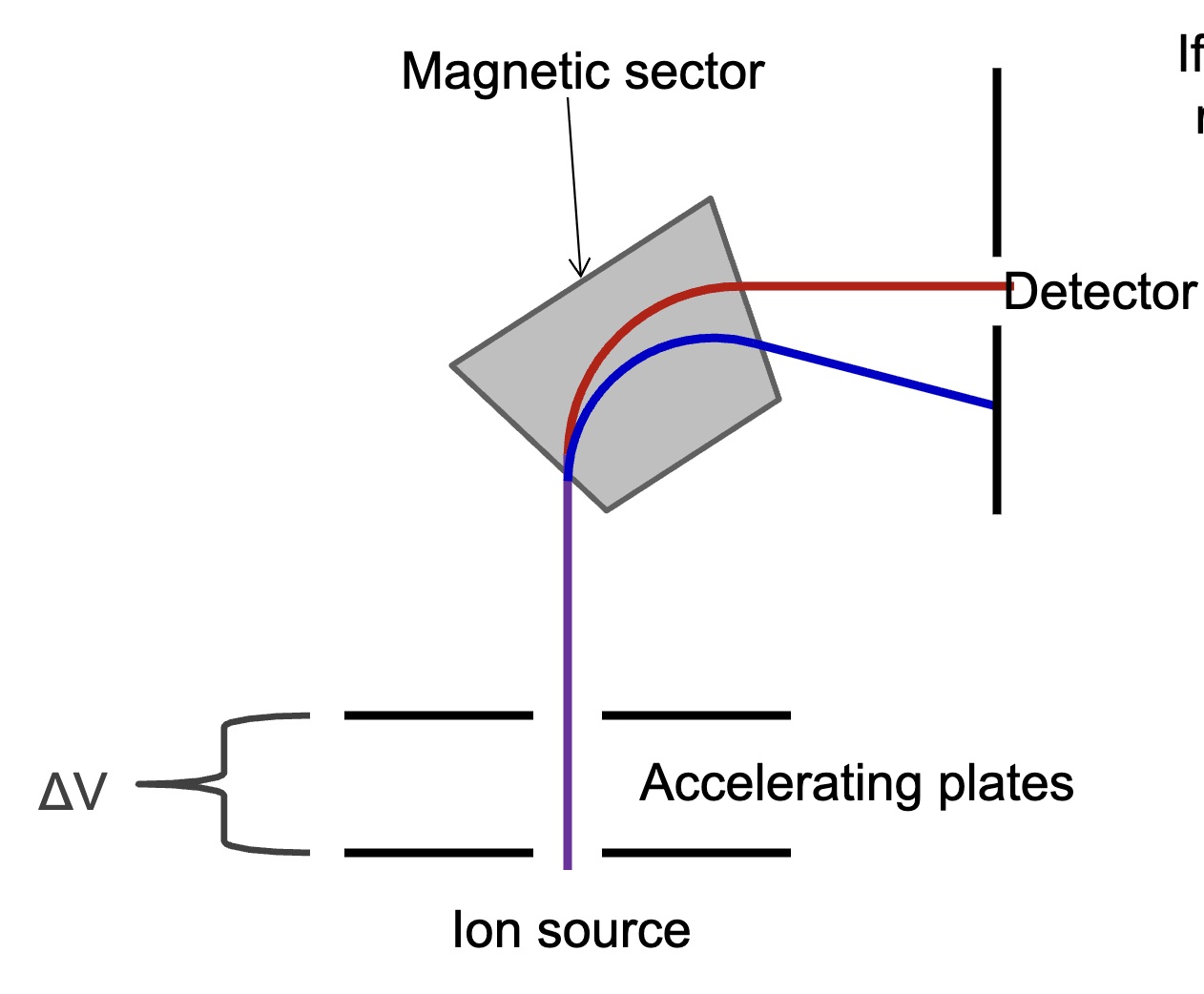
How can one select a specific M/Z in mass spec? What precautions must be taken?
Keep either magnetic field strength or voltage constant while varying the other. However, varying voltage is not ideal because it effects the transmission and detection of the ions. Ideally, one would want to vary the magnetic field but this must be done slowly because it reflects a 10-fold change.
What is a double-focusing mass spec and why is it important? Draw one.
A double-focusing mass spec combines a magnetic and an electric sector that compensates for the initial conditions of ions. Before acceleration, ions have kinetic energy which causes changes in radius of curvature and initial direction. This results in peak broadening, limiting the resolving power. The electric sector eliminates ions with energy far from the desired. In combintaion with the magnetic sector, only one mass is focused at a time, increasing resolution.
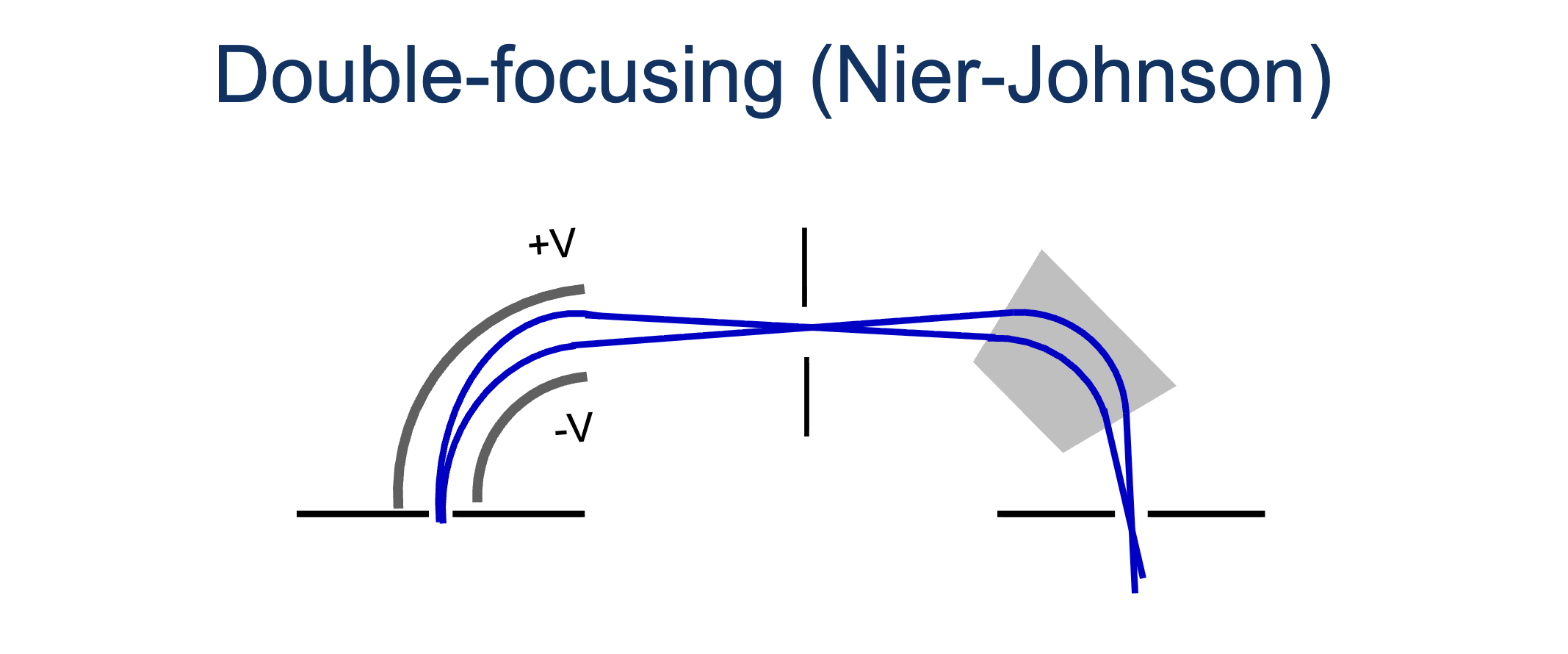
Nier-Johnson double focusing vs Mattauch-Herzog double focusing
Nier-Johnson scans one M/Z at a time and the different sectors are not in the same plane. Mattauch-Herzog - all M/Z are scanned at once along the same plane, usually uses a spectograph.
What is the field-free region length and how does it effect resolution?
The field-free region length (L) is the distance between the acceleration plates and the detector. The longer the length, the higher the resolution.
Describe time of flight spectroscopy.
The sample is ionized and accelerated through an electric field. The ions travel down the field-free region and reach the detector. The M/Z is measured by how long it takes the ions to reach the detector.
What is a problem with time of flight spectroscopy and how can it be corrected?
The initial energy of the ions effects its total energy after acceleration, causing ions of the same M/Z to reach the detector at different times. A reflectron can be use to equate the energy of the ions. A reflectron contains electrostatic ion mirrors that reflect ions back towards the detector. Ions with greater energy will penetrate the reflectron deeper, slowing them down and synchronizing arrival times of the ions. Additionally, path length is also elongated.
How does quadrupole mass spectroscopy work?
Quadrupole MS filters ions along a path. Ions are injected along the center between the rods. The electric fields cause the ions to oscillate. The ions of stable oscillations make it through and the unstable oscillations will hit the rod and be filtered out.
What is a high pass filter in quadrupole MS?
Ions are pushed to the center by the DC field (constant voltage) and pulled towards the rods on a negative ½ cycle by the AC field. Light ions react quickly and strike the rods. Heavy ions react slowly so the AC direction switches before they can strike rod. Therefore, a high pass filter filters out light ions and heavy ions reach the detector.
What is a low pass filter in quadrupole MS?
Ions are pulled towards the rods by the DC field (constant voltage) and pushed from the rods on a positive ½ cycle by the AC field. Light ions react quickly and are pulled away before they strike the ride. Heavy ions react slowly so they strike the rod before the AC direction change. Therefore, a low pass filter filters out heavy ions and heavy and light ions reach the detector.
Advantages and disadvantages of quadrupole MS
Advantages
high signal due to large apertures
can operate at high pressure due to short pathlength
fast scanning due to short pathlength and electric field can be switched quickly
fast and sensitive
Disadvantages
moderate resolution
How does ion trap mass spectroscopy work?
Ions are stored in place by AC electric field. Ions of stable oscillation will stay and ions of unstable oscillation will escape. Increasing the AC voltage ejects the unstable ions one by one in order of M/Z.
What is the space charge effect and why is it important?
When multiple ions are held in one place, their charges effect one another. This limits trap capacity and effects resolution.
How does FTICR work?
Ions enter an magnetic field and move in a circular orbit with lighter ions moving faster and creating a higher frequency orbit. The motion of the ions create a current (AC), outputting various frequencies. These frequencies are converted to M/Z via FT.
What is acquisition time and why is it important?
The amount of time the instrument takes recording the ion signal after excitation. Important because the longer the acquisition time, the higher the resolution.
How does orbitrap mass spec work and how is it different/similar to FTICR?
Ions are trapped electrostatically (unlike FTICR - magnetically) and oscillate left and right depending on M/Z. The induced current on the outer elctrodes are transmitted and converted to M/Z using FT (like FTICR).
Rank the mass spec analyzers from highest resolving power the lowest.
FT-ICR > orbitrap = double focusing > TOF > ion trap > mag. sector = quad.
Rank the mass spec analyzers from highest mass range to lowest.
TOF > FT-ICR > orbitrap = double-foc. = ion trap > quad.
Rank the mass spec analyzers from highest dynamic range to lowest.
double-foc > quad > TOF = ion trap = FTICR = orbitrap
Rank the mass spec analyzers from highest cost to lowest.
FT-ICR > orbitrap > double-foc. >TOF > ion trap = quad
Rank the mass spec analyzers from fastest to slowest.
TOF > double-foc (spectograph) > quad. = ion trap > double-foc. = FT-ICR = orbitrap
Rank the mass spec analyzers from highest pressure tolerance to lowest.
ion trap > quad > double-foc. = TOF > FTICR
Rank the mass spec analyzers from largest to smallest.
double-foc = FT-ICR > TOF = orbitrap > quad = ion trap
What is ICP and how does it work? What are its advantages and disadvantages?
ICP (inductively coupled plasma) is a type of mass spec ion source. The sample is typically introduced through nebulization, vapor generation, or laser ablation. A high temperature plasma (ionized argon gas) breaks down the sample into constituent atoms.
Advantage
low LOD (ppt)
Disadvantage
sample is completely atomized - loses structural/molecular information
What is glow discharge ionization and how does it work?
Type of mass spec ion source used for solid samples. An electric current is run through argon gas in which the argon is ionized and a plasma forms. The argon ions charge toward the solid (sputtering) to move the solid into the gas phase. The gas particles then enter the plasma and are ionized.
What is thermal ionization and how does it work? What mass analyzer does is it usually paired with? What are some of its advantages and disadvantages?
Type of mass spec ion source. The sample is placed on a filament and an electrical current is passed through. The sample is vaporized and some of the atoms are ionized. Usually paired with a magnetic sector.
Advantages
high resolution
better precision and accuracy for isotope ratio
inexpensive
Disadvantages
not applicable to all elements
slow
What is electron-impact ionization and how does it work? What mass analyzer does it usually use? What are some advantages and disadvantages of it?
Type of mass spec ion source. A heated filament emits high-energy electron at incoming gas-phase molecules. This results in the ejection of some electrons from the molecules, creating an unstable ion, leading to fragmentation. Usually paired with a quadrupole.
Advantage
fragmentation can help identify structure of molecule beyond molecular weight
Disadvantage
limited mass range (requires volatile analyte)
What is fragmentation and why is it important?
Fragmentation occurs when unstable ions break apart into smaller ions. Important because it reveals structural information beyond just molecular weight.
What is chemical ionization and how does it work? What is an advantage and disadvantage?
Type of mass spec ion source. Reagent gas (CH4, NH3) fills ion chamber. Electrons collide with gas and gas transfers proton to analyte.
Advantage
soft ionization = reduced fragmentation = preserves analyte
Disadvantage
limited structural information
What is matrix-assisted laser desorption ionization (MALDI) and how does it work? What mass analyzer is it used with? What are some of its advantages and disadvantages?
Type of mass spec ion source. Sample is deposited in matrix and a laser strikes the matrix. Energy is transferred from matrix to analyte, donating a charge (usually proton) to analyte. Almost always used with time of flight mass analyzer.
Advantages
reduced fragmentation
capable of ionizing and vaporizing high-mass molecules (proteins)
ion production is pulsed
infinite mass range
Disadvantage
limited structural information
What is electron-spray ionization and how does it work. What are some of its advantages?
Type of mass spec ion source. Sample is introduced in solution. The solution flows through metal capillary tube and voltage is applied, creating a spray of droplets holding charge. The solvent evaporates and the droplets shrink until the droplet breaks apart leaving the multiply-charged analyte.
Advantages
efficient for measuring proteins using quadrupole because M/Z is so low (high mass, high charge)
redundant measurements = high mass accuracy
What is desorption electron-spray ionization and how does it work?
Type of mass spec ion source. Charged particles are sprayed onto sample surface. The molecules on surface are desorbed (lifted off) and enter gas phase. In the process, the molecules pick up charge from the droplets.
What is the faraday cup? What mass analyzers are commonly used with it? What are its advantages and disadvantages?
A type of mass spec detector. Commonly used with magnetic sector and double-focusing
Advantages
signal only depends on charge
not affected by magnetic field
Disadvantage
slow
What is an electron multiplier and how does it work? What mass analyzer is it commonly used with? What are its advantages and disadvantages?
A type of mass spec detector. It uses dynodes to amplify the electrons. Often used with quadrupole.
Advantages
fast
provides gain (signal amplification, sensitivity)
Disadvantages
uneven response (mass and size-sensitive)
What are type of mass analyzers are used with array detectors?
Spectograph-type mass analyzers (TOF, mattauch-herzog double-focusing)
How does chromotography work?
An analyte is separated through interactions between the mobile and stationary phases.

What does each variable mean?
N = # of theoretical plates
L = length of column (cm)
H = height of theoretical plates (cm)
Significance of theoretical plates
Increase in theoretical plates increases the column’s efficiency

What does each variable mean?
N = # of plates
TR = retention time
W = width of peak
W1/2 = full width at half max (FWHM)
What does retention factor measure?
How strongly analyte is retained by stationary phase
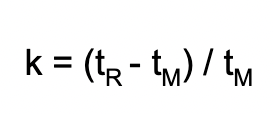
What does each variable mean?
k = retention factor
tR = retention time (time in mobile phase and stationary phase)
tM = time in mobile phase