Chemistry - PE Unit 4 Quantum Numbers
1/17
Earn XP
Description and Tags
If you have the concept and it isn't exactly what it wants, just override it from being wrong.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
18 Terms
What does the Principle Quantum Number tell you? What is the ___?
Tells how far an electron is from the nucleus. n
Explain the Principle Quantum Number
The larger the n, the further away the electron is from the nucleus, creating less attraction forces towards the nucleus. Outside electrons are likely to be the “bonding” electrons called valence electrons. (valence e-)
What is another thing that an energy level is called?
A shell
What does the Orbital Quantum Number tell you? What is the ___?
Describes the shape of the Orbital. Sublevels
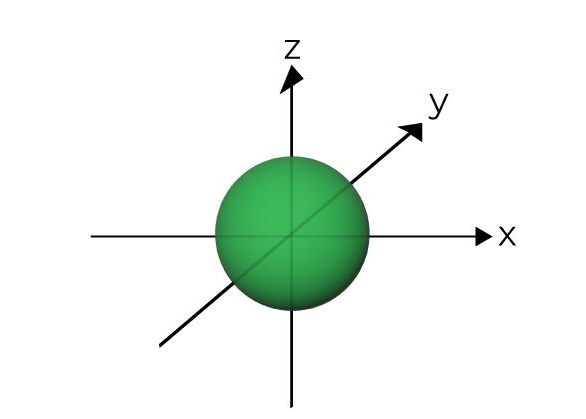
What is the name of this?
s orbital
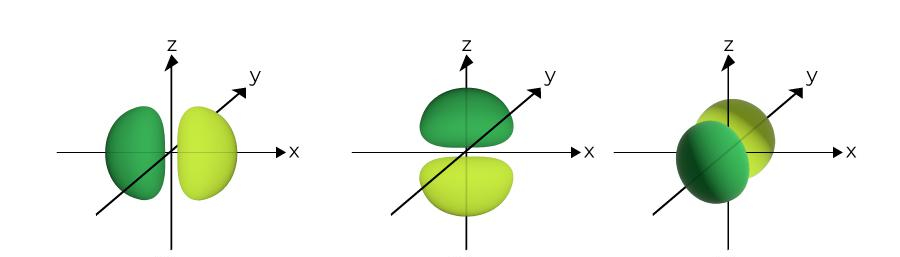
What is the name of this?
p orbital
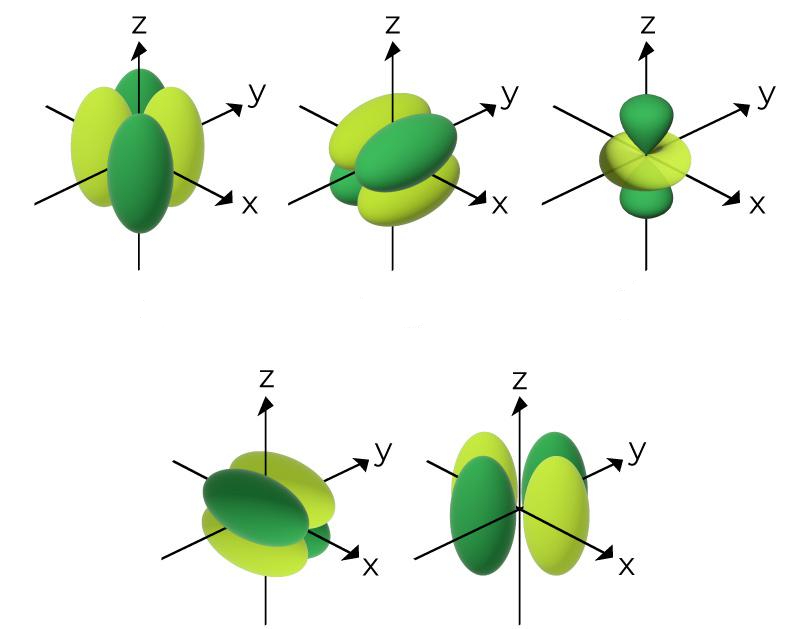
What is the name of this?
d orbital
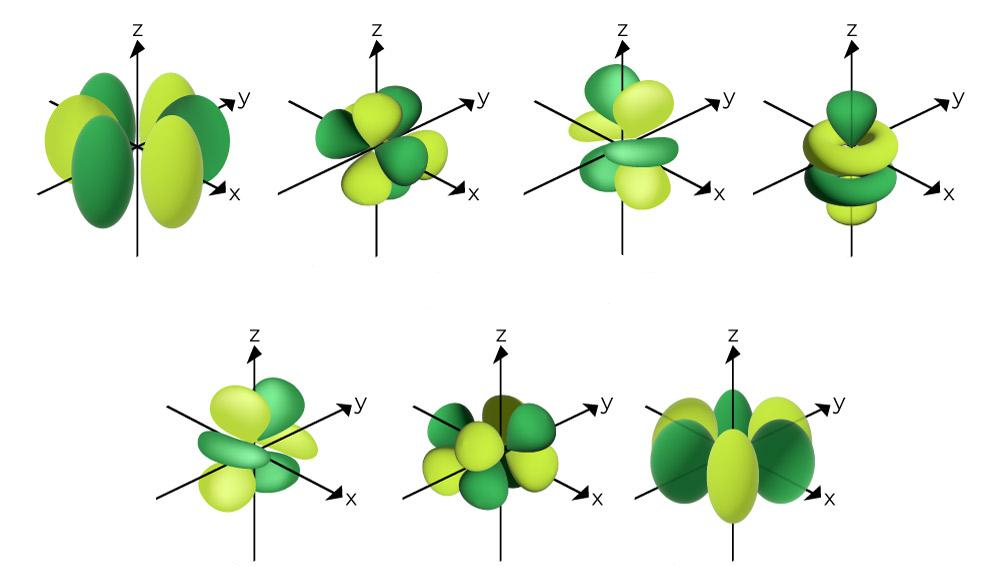
What is the name of this?
f orbital
What does Orientation Quantum Number tell you?
Describes how the Orbital is arranged/positioned in space
How many orientations does each orbital have? Write like s = #, p = #, etc.
s = 1, p = 3, d = 5, f = 7
What does Spin Quantum Number tell you?
Describes the direction the electron (e-) is spinning
Explain the Pauli Exclusion Principle
Only 2 electrons can be in the same orbital and they much have opposite spins
What do the Electron Configurations tell you?
Describes where all the electrons in an atom are located
Explain the Aufbau Principle
Electrons occupy the orbitals of lowest energy first
Explain the Hunds Rule
Electrons occupy the orbitals of the same energy with the same spin, until they have to double up
What would you write for a single electron?
Up arrow or down arrow (↑) or (↓)
What would you write for an electron pair?
A half up arrow and half down arrow (↑↓)
Practice being able to read short hand notation. What element is this: 1s22s22p63s23p3
Phosphorus