Topic 7 - rates of reaction and energy changes
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
16 Terms
Core Practical: Investigate the effects of changing the conditions of a reaction on the rates of chemical reactions by: measuring the production of a gas (in the reaction between hydrochloric acid and marble chips) and observing a colour change (in the reaction between sodium thiosulfate and hydrochloric acid)
in these experiments, you are investigating the effect on rate of changing the size of the marble chips (smaller chips = larger surface area = faster rate) and also the effect of changing the concentration of hydrochloric acid (greater concentration = greater number of particles in a given volume = faster rate)
method
support a gas syringe with a stand, boss and clamp
using a measuring cylinder, add 50cm3 of dilute hydrochloric acid to a conical flask
add 0.4g of calcium carbonate to the flask. immediately connect the gas syringe and start a stop clock
record the time for every 10cm3 of gas produced
when the reaction is complete, clean the apparatus as directed by the teacher
repeat steps 1 to 5 with different concentrations of hydrochloric acid
hazards, risks and precautions
hot hydrochloric acid - causes skin and eye irritation and burns to the skin, wear gloves and eye protection and do not heat above 60°C
fizzing in the reaction mixture - spray or foam escaping which may damage skin and eyes, use a large conical flask so there is plenty of space inside and do not look over the top when adding the calcium carbonate
suggest practical methods for determining the rate of a given reaction
rates of reactions can be measure using the amount of product used, or amount of product formed over time:
rate of reaction = amount of reactant used/time
rate of reaction = amount of product formed/time
quantity of reactant or product can be measured by the mass in grams or by a volume in cm3
units of rate of reaction may be given as g/s or cm3/s
to measure reactant used: if the product is a gas, which will be given off, you can carry out the reaction on a set of weighing scales and measure how much mass is lost
to measure product formed: if the product is a gas, you can measure the volume of gas produced in a gas syringe
explain how reactions occur
chemical reactions only occur when the reacting particles collide with enough energy, the minimum amount of energy required is called the activation energy
in order to increase the rate of reaction, you need to increase the frequency/energy of collisions, so that more of them reach the activation energy
this can be done by: increasing temperature, pressure, concentration, surface area or by using a catalyst
explain the effects on rates of reactions of changes in temperature, concentration, surface area to volume ratio of a solid and pressure (on reactions involving gases) in terms of frequency and/or energy of collisions between particles
increasing the temperature increases the rate of reaction. as increasing temperature increases the kinetic energy of particles, so they collide more frequently and energetically
increasing pressure in reacting gases increases the rate of reaction, as it increases the number of particles in a given volume so increases the frequency of collisions
increasing concentration of reacting solutions increases the rate of reaction, as it increases the number of particles in a given volume and so increases the frequency of collisions
increasing the surface area of solid reactants increases the rate of reaction, as it increases the frequency of collisions so increases the rate of reaction
interpret graphs of mass, volume or concentration of reactant or product against time
to time rate reaction graphically
draw tangents to curves and use the slope of the tangent as a measure of the rate of reaction
gradient = rate of reaction, therefore use this information to interpret any given graph, therefore a steeper line means a greater increase/decrease in rate
remember to check if the graph is showing you a product or a reactant - for a product you would be expecting the mass/volume/concentration to increase, whereas for a reactant you would be expecting the mass/volume/concentration to decrease
describe a catalyst as a substance that speeds up the rate of a reaction without altering the products of the reaction, being itself unchanged chemically and in mass at the end of the reaction
catalysts are substances that speed up chemical reactions without being changed or used up during the reaction. they are the same and have the same mass at the end of the reaction
explain how the addition of a catalyst increases the rate of a reaction in terms of activation energy
catalysts decrease the activation energy; this increases the proportion of particles with energy to react, leading to more frequent successful collisions and so an increased rate of reaction
catalysts lower the activation energy by providing a different pathway for a chemical reaction that has a lower activation energy
what are enzymes
enzymes act as catalysts in biological systems
yeast is the enzyme used in the production of ethanol as it is fermented from sugars, ethanol is in alcoholic drinks
what do changes in heat energy accompany
salts dissolving in water is either exothermic or endothermic
neutralisation reaction is exothermic
displacement is an exothermic or endothermic reaction
precipitation is an exothermic reaction
if these reactions take place in a solution, you can carry them out in a polystyrene cup with a lid, and measure the temperature change using a thermometer
what is an exothermic change or reaction
an exothermic is one that transfers energy to the surroundings so the temperature of the surroundings increases
examples of exothermic reaction include; combustion, many oxidation reactions and neutralisation
everyday examples of exothermic reactions include; self-heating cans (eg. for coffee) and hand warmers
describe an endothermic change or reaction
an endothermic reaction is one that takes in energy from the surroundings so the temperature of the surroundings decreases
examples of endothermic reactions are thermal decomposition and the reaction of citric acid and sodium hydrogen carbonate
some sports injury packs are based on endothermic reactions
what type of reaction is the breaking bonds
breaking of bonds is endothermic, and the making of bonds is exothermic
during a chemical reaction:
energy must be SUPPLIED to BREAK bonds in the reactants
energy is RELEASED when bonds in the products are FORMED
what is the overall heat energy change for a reaction
energy taken in to break > energy released when formed = ENDOTHERMIC (because overall energy has been taken in)
energy taken in to break < energy released when formed = EXOTHERMIC (because overall energy has been released)
calculate the energy change in a reaction given the energies of bonds (in kJ mol-1)
the energy needed to break bonds and energy released when bonds are formed can both be calculated from bonds energies
sum of energy taken in to break bonds - sum of energy released to form bonds = overall energy change
if the energy out > energy in, the energy change will be negative showing an exothermic reaction and if the energy out < energy in, the energy change will be positive showing an endothermic reaction
explain the term activitation energy
chemical reactions can occur only when reacting particles collide with each other and with sufficient energy
activation energy = minimum amount of energy that particles must have to react
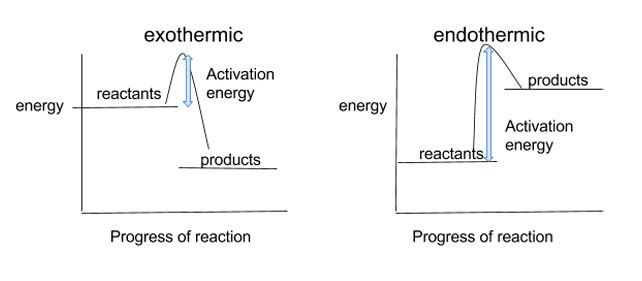
draw and label reaction profiles for endothermic and exothermic reactions, identifying activation energy
reaction profiles can be used to shows the relative energies of reactants and products, the activation energy and the overall energy change of a reaction
for the exothermic diagram, the products have less energy than the reactants, because the energy has been released to the surroundings
for the endothermic diagram, the reactants have less energy than the products, because the energy has been taken in from the surroundings