DF1-
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
10 Terms
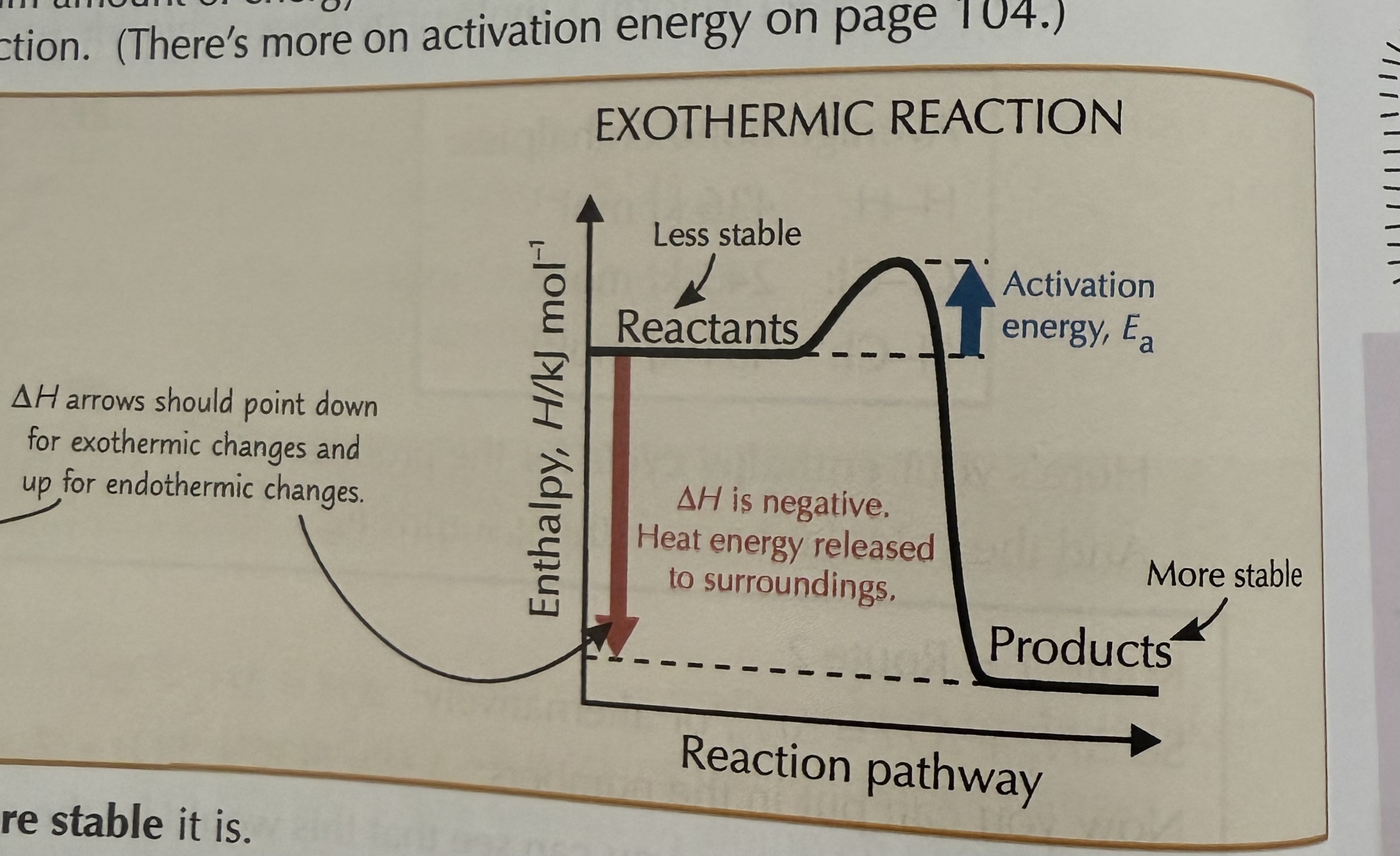
Exothermic reaction
A chemical reaction that transfers heat energy into its surroundings
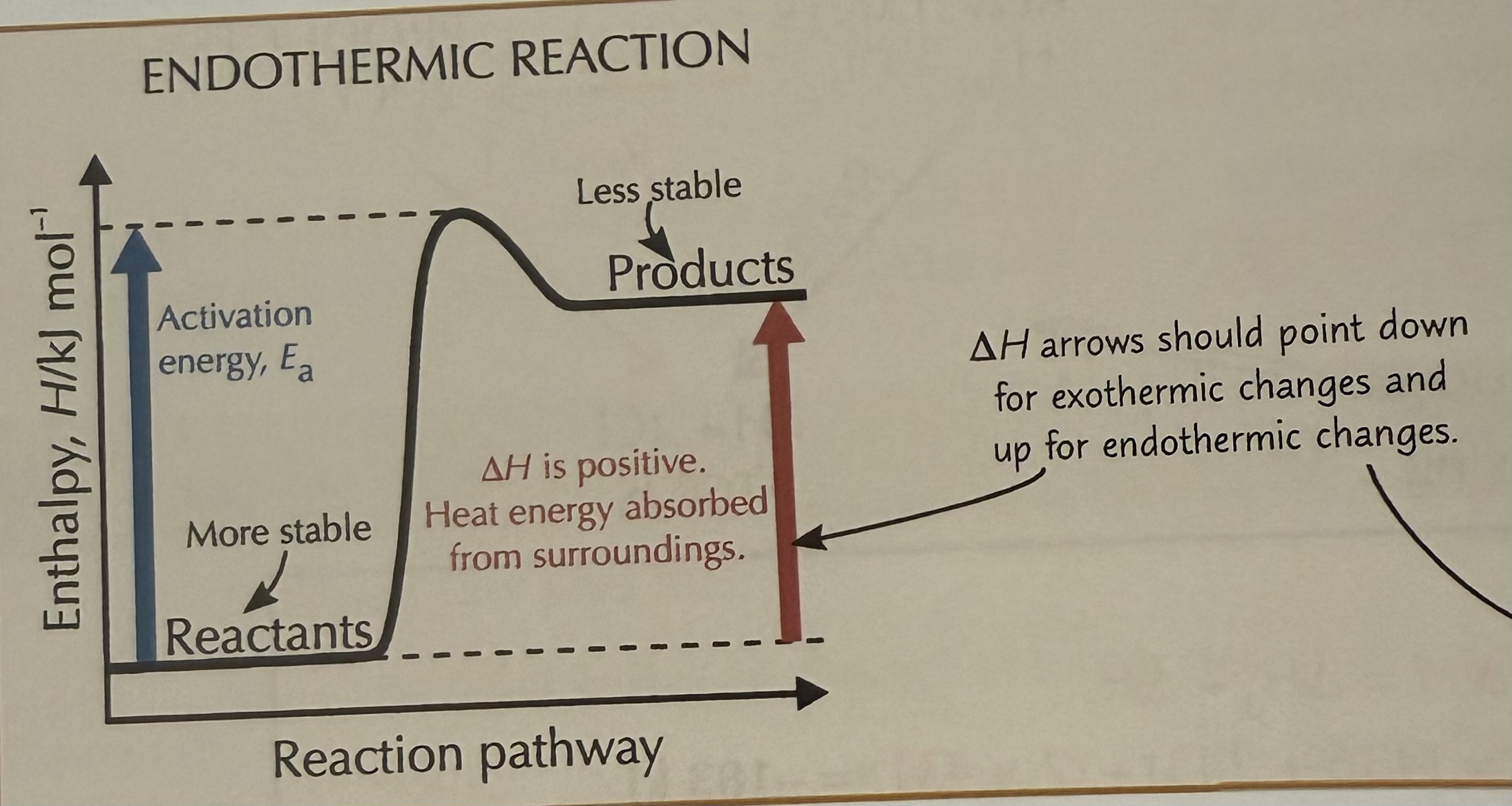
Endothermic reaction
A chemical reaction that absorbs energy from its surroundings
What are standard conditions?
1 atmosphere pressure (101 kPa)
temperature - 298k (25•c)
concentration of solutions involved. 1 moldm-3 for any solutions
Standard enthalpy change of a reaction
The enthalpy change when molar quantities of reactants as stated in the equation react together under standard conditions
Standard enthalpy change of combustion
The enthalpy change when 1 mole of a substance burns completely in oxygen under standard conditions
Standard enthalpy change of formation
The enthalpy change when 1 mole of a compound is formed from its elements under standard conditions in standard states
Standard enthalpy change of neutralisation
The enthalpy change when 1 mole of hydrogen ions react with 1 mole of hydroxide ions to form 1 mole of water under standard conditions and in solutions with a concentration of 1 moldm-3
What is the equation to measure enthalpy changes?
Energy transferred (kJ) = specific heat capacity x mass x temperature change
What is the specific heat capacity of water?
4.18
How to calculate enthalpy change in solution
Energy transferred equation/1000
Calculate moles using conc x volume
Divide the energy transferred/ moles
Round to nearest full number