Chem Exam Review Stuff!
5.0(2)
Card Sorting
1/23
Last updated 3:03 PM on 1/17/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
24 Terms
1
New cards
anion
negative
2
New cards
cation
positive
3
New cards
alpha release
number -2, mass -4
4
New cards
beta release
number +1, mass stays
5
New cards
which color on the visible spectrum has the highest energy?
violet
6
New cards
which color on the visible spectrum has the lowest energy?
red
7
New cards
"sea of electrons" (electrons float around between nuclei) is a characteristic of a...
metal
8
New cards
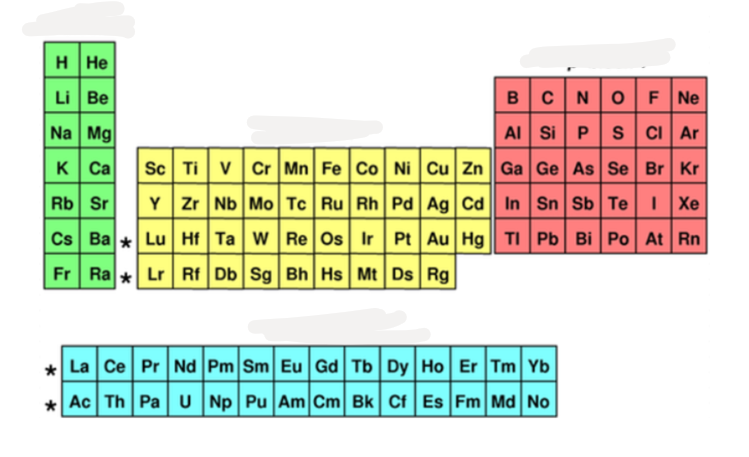
what block is %%green%%?
s
9
New cards
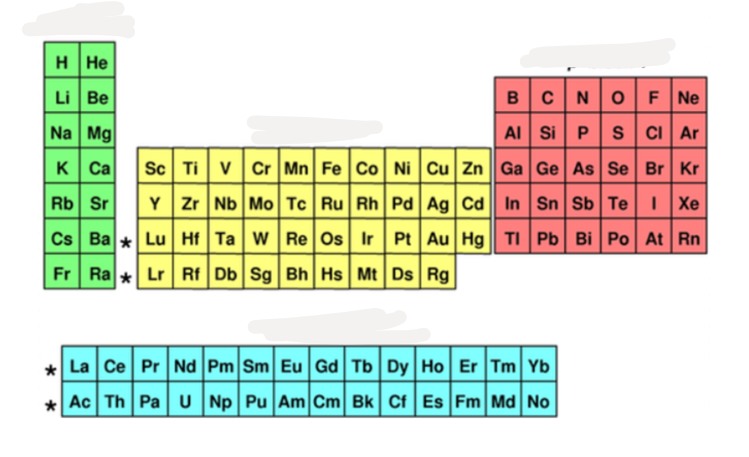
what block is yellow?
d
10
New cards
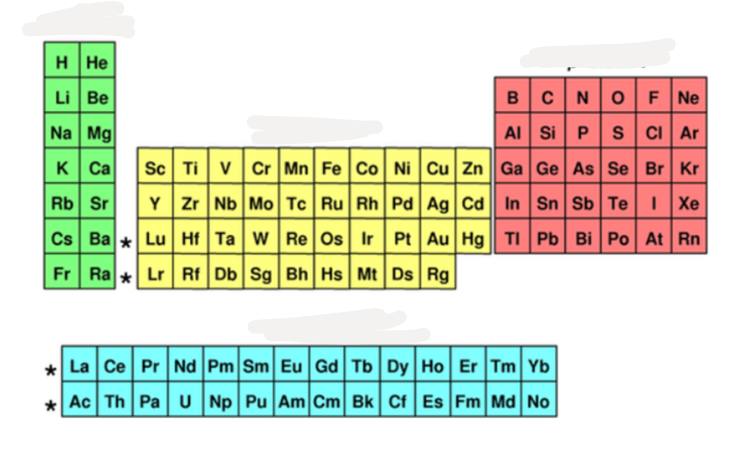
what block is @@red@@?
p
11
New cards
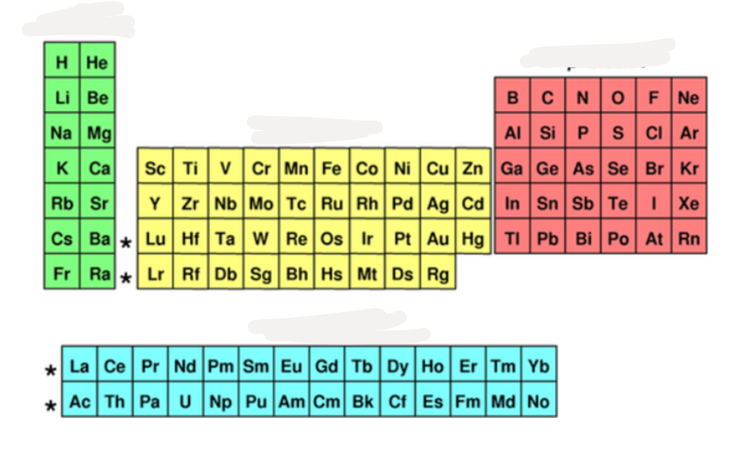
what block is ^^teal^^?
f
12
New cards
metal and nonmetals have a ____ bond
ionic
13
New cards
nonmetals and nonmetals have a ____ bond
covalent
14
New cards
metals and metals have a ____ bond
metallic
15
New cards
it takes the most energy to break a ____ bond
covalent
16
New cards
high melting point, high bioling point, high conductivity, malleability and ductility are characteristics of ____ bonding
metallic
17
New cards
water has a _____ bond
polar
18
New cards
the total oxidation state of a compound must always equal
0
19
New cards
the elements with the **lowest** ionization energies are…
noble gases
20
New cards
acid/base neutralization reactions are…
double replacement
21
New cards
if reactants are **taken away**, the equation shifts
to the left
22
New cards
if reactants are **added**, the equation shifts
to the right
23
New cards
acidity is measured by how much…
H+
24
New cards
base is measured by how much…
OH-